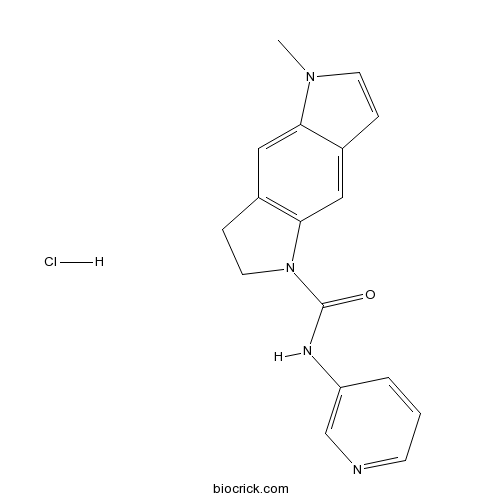
SB 206553 hydrochloridePotent, selective 5-HT2C/5-HT2B antagonist. Orally active CAS# 1197334-04-5 |
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- BIRB 796 (Doramapimod)
Catalog No.:BCC2535
CAS No.:285983-48-4
- TAK-715
Catalog No.:BCC3968
CAS No.:303162-79-0
- PH-797804
Catalog No.:BCC3672
CAS No.:586379-66-0
- LY2228820
Catalog No.:BCC2528
CAS No.:862507-23-1
- SB 203580 hydrochloride
Catalog No.:BCC4293
CAS No.:869185-85-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1197334-04-5 | SDF | Download SDF |
| PubChem ID | 11957707 | Appearance | Powder |
| Formula | C17H17ClN4O | M.Wt | 328.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | 1-methyl-N-pyridin-3-yl-6,7-dihydropyrrolo[2,3-f]indole-5-carboxamide;hydrochloride | ||
| SMILES | CN1C=CC2=CC3=C(CCN3C(=O)NC4=CN=CC=C4)C=C21.Cl | ||
| Standard InChIKey | VGEMBOFBPSNOIO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H16N4O.ClH/c1-20-7-4-12-10-16-13(9-15(12)20)5-8-21(16)17(22)19-14-3-2-6-18-11-14;/h2-4,6-7,9-11H,5,8H2,1H3,(H,19,22);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

SB 206553 hydrochloride Dilution Calculator

SB 206553 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0414 mL | 15.2068 mL | 30.4136 mL | 60.8273 mL | 76.0341 mL |
| 5 mM | 0.6083 mL | 3.0414 mL | 6.0827 mL | 12.1655 mL | 15.2068 mL |
| 10 mM | 0.3041 mL | 1.5207 mL | 3.0414 mL | 6.0827 mL | 7.6034 mL |
| 50 mM | 0.0608 mL | 0.3041 mL | 0.6083 mL | 1.2165 mL | 1.5207 mL |
| 100 mM | 0.0304 mL | 0.1521 mL | 0.3041 mL | 0.6083 mL | 0.7603 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SDZ 205-557 hydrochloride
Catalog No.:BCC7246
CAS No.:1197334-02-3
- Sazetidine A dihydrochloride
Catalog No.:BCC7468
CAS No.:1197329-42-2
- TGR5 Receptor Agonist
Catalog No.:BCC4195
CAS No.:1197300-24-5
- Baohuoside VII
Catalog No.:BCN2889
CAS No.:119730-89-1
- Fupenzic acid
Catalog No.:BCN6085
CAS No.:119725-20-1
- 2-Epitormentic acid
Catalog No.:BCN6084
CAS No.:119725-19-8
- UNC 0224
Catalog No.:BCC2430
CAS No.:1197196-48-7
- Sutchuenmedin A
Catalog No.:BCN6854
CAS No.:1197194-31-2
- PF-05212384 (PKI-587)
Catalog No.:BCC4987
CAS No.:1197160-78-3
- 5-Methylfurmethiodide
Catalog No.:BCC6707
CAS No.:1197-60-0
- 4-Aminophenylacetic acid
Catalog No.:BCC8687
CAS No.:1197-55-3
- Tranexamic acid
Catalog No.:BCN2710
CAS No.:1197-18-8
- Schizanthine G
Catalog No.:BCN1938
CAS No.:119736-74-2
- Schizanthine M
Catalog No.:BCN1939
CAS No.:119736-78-6
- Tautomycetin
Catalog No.:BCC7320
CAS No.:119757-73-2
- Baohuoside VI
Catalog No.:BCC8129
CAS No.:119760-73-5
- 29-Norlanosta-8,24-diene-1alpha,2alpha,3beta-triol
Catalog No.:BCN7984
CAS No.:119765-92-3
- 3-Furfuryl 2-pyrrolecarboxylate
Catalog No.:BCN6086
CAS No.:119767-00-9
- AP26113
Catalog No.:BCC1069
CAS No.:1197958-12-5
- Mirin
Catalog No.:BCC5986
CAS No.:1198097-97-0
- Sodium Channel inhibitor 1
Catalog No.:BCC1959
CAS No.:1198117-23-5
- Cerdulatinib (PRT062070)
Catalog No.:BCC8068
CAS No.:1198300-79-6
- Coptisine sulfate
Catalog No.:BCN2286
CAS No.:1198398-71-8
- Phyperunolide E
Catalog No.:BCN7292
CAS No.:1198400-52-0
5-HT2A and 5-HT2C/2B receptor subtypes modulate dopamine release induced in vivo by amphetamine and morphine in both the rat nucleus accumbens and striatum.[Pubmed:11850146]
Neuropsychopharmacology. 2002 Mar;26(3):311-24.
In vivo microdialysis and single-cell extracellular recordings were used to assess the involvement of serotonin(2A) (5-HT(2A)) and serotonin(2C/2B) (5-HT(2C/2B)) receptors in the effects induced by amphetamine and morphine on dopaminergic (DA) activity within the mesoaccumbal and nigrostriatal pathways. The increase in DA release induced by amphetamine (2 mg/kg i.p.) in the nucleus accumbens and striatum was significantly reduced by the selective 5-HT(2A) antagonist SR 46349B (0.5 mg/kg s.c.), but not affected by the 5-HT(2C/2B) antagonist SB 206553 (5 mg/kg i.p.). In contrast, the enhancement of accumbal and striatal DA output induced by morphine (2.5 mg/kg s.c.), while insensitive to SR 46349B, was significantly increased by SB 206553. Furthermore, morphine (0.1-10 mg/kg i.v.)-induced increase in DA neuron firing rate in both the ventral tegmental area and the substantia nigra pars compacta was unaffected by SR 46349B (0.1 mg/kg i.v.) but significantly potentiated by SB 206553 (0.1 mg/kg i.v.). These results show that 5-HT(2A) and 5-HT(2C) receptors regulate specifically the activation of midbrain DA neurons induced by amphetamine and morphine, respectively. This differential contribution may be related to the specific mechanism of action of the drug considered and to the neuronal circuitry involved in their effect on DA neurons. Furthermore, these results suggest that 5-HT(2C) receptors selectively modulate the impulse flow-dependent release of DA.
In vitro and in vivo profile of SB 206553, a potent 5-HT2C/5-HT2B receptor antagonist with anxiolytic-like properties.[Pubmed:8821530]
Br J Pharmacol. 1996 Feb;117(3):427-434.
1. SB 206553 (5-methyl-1-(3-pyridylcarbamoyl)-1,2,3,5-tetrahydropyrrolo[2 ,3-f]indole) displays a high affinity (pK1 7.9) for the cloned human 5-HT2C receptor expressed in HEK 293 cells and the 5-HT2B receptor (pA2 8.9) as measured in the rat stomach fundus preparation. SB 206553 has low affinity for cloned human 5-HT2A receptors expressed in HEK 293 cells (pK1 5.8) and (pK1 < 6) for a wide variety of other neurotransmitter receptors. 2. SB 206553 appears to be a surmountable antagonist of 5-HT-stimulated phosphoinositide hydrolysis in HEK 293 cells expressing the human 5-HT2C receptor (pKB 9.0). 3. The compound potently (ID50 5.5 mg kg-1, p.o., 0.27 mg kg-1, i.v.) inhibited the hypolocomotor response to m-chlorophenylpiperazine (mCPP), a putative model of 5-HT2C/5-HT2B receptor function in vivo. 4. At similar doses (2-20 mg kg-1, p.o.) SB 206553 increased total interaction scores in a rat social interaction test and increased punished responding in a rat Geller-Seifter conflict test. These effects are consistent with the possession of anxiolytic properties. 5. SB 206553 also increased suppressed responding in a marmoset conflict model of anxiety at somewhat higher doses (15 and 20 mg kg-1, p.o.) but also reduced unsuppressed responding. 6. These results suggest that SB 206553 is a potent mixed 5-HT2C/5-HT2B receptor antagonist with selectivity over the 5-HT2A and all other sites studied and possesses anxiolytic-like properties.
5-Methyl-1-(3-pyridylcarbamoyl)-1,2,3,5-tetrahydropyrrolo[2,3-f]indole: a novel 5-HT2C/5-HT2B receptor antagonist with improved affinity, selectivity, and oral activity.[Pubmed:7629791]
J Med Chem. 1995 Jul 7;38(14):2524-30.
The preparation of a series of conformationally restricted analogues of indolylurea 1, namely tetrahydropyrroloindoles and tetrahydropyrroloquinolines, is described. The binding affinities of these compounds at 5-HT2A, 5-HT2B, and 5-HT2C receptors were determined. Of these compounds, the 1,2,3,5-tetrahydropyrrolo[2,3-f]indole derivative, compound 11, was found to have high affinity for the 5-HT2C (pKI 8.0) and 5-HT2B receptors (pA2 8.5), with excellent selectivity over the 5-HT2A and various other receptors (pKI < 6). 11 is also considerably more active than 1 in both an in vitro functional model, 5-HT-stimulated phosphoinositol hydrolysis (pKB 8.8), and an in vivo functional model, mCPP-induced hypolocomotion (ID50 5.5 mg/kg po). 11 should therefore be of significant utility as a pharmacological tool to delineate the functional significance of blockade of 5-HT2B and 5-HT2C receptors.


