TAK-715p38 MAPK inhibitor CAS# 303162-79-0 |
- Skepinone-L
Catalog No.:BCC1953
CAS No.:1221485-83-1
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- SB 239063
Catalog No.:BCC1923
CAS No.:193551-21-2
- SD-06
Catalog No.:BCC1937
CAS No.:271576-80-8
- LY2228820
Catalog No.:BCC2528
CAS No.:862507-23-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 303162-79-0 | SDF | Download SDF |
| PubChem ID | 9952773 | Appearance | Powder |
| Formula | C24H21N3OS | M.Wt | 399.52 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (250.31 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
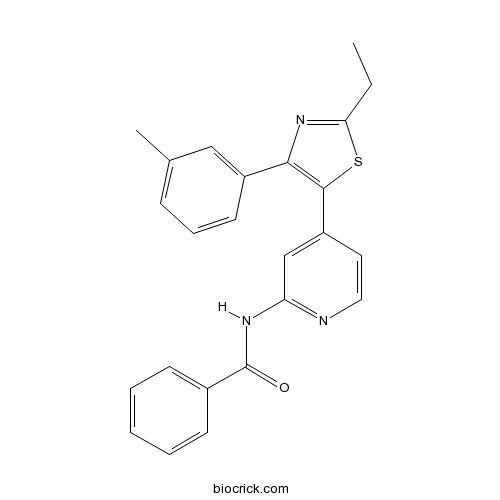
| Chemical Name | N-[4-[2-ethyl-4-(3-methylphenyl)-1,3-thiazol-5-yl]pyridin-2-yl]benzamide | ||
| SMILES | CCC1=NC(=C(S1)C2=CC(=NC=C2)NC(=O)C3=CC=CC=C3)C4=CC(=CC=C4)C | ||
| Standard InChIKey | HEKAIDKUDLCBRU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H21N3OS/c1-3-21-27-22(18-11-7-8-16(2)14-18)23(29-21)19-12-13-25-20(15-19)26-24(28)17-9-5-4-6-10-17/h4-15H,3H2,1-2H3,(H,25,26,28) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of p38 MAPK (IC50 = 7.1 nM for p38 MAPKα). Also inhibits Wnt-3a-stimulated β-catenin signaling. Inhibits 22 kinases by more than 80%, including CK1δ/ε. Anti-inflammatory and anti-rheumatoid arthritis agent. Orally bioavailable. |

TAK-715 Dilution Calculator

TAK-715 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.503 mL | 12.515 mL | 25.03 mL | 50.0601 mL | 62.5751 mL |
| 5 mM | 0.5006 mL | 2.503 mL | 5.006 mL | 10.012 mL | 12.515 mL |
| 10 mM | 0.2503 mL | 1.2515 mL | 2.503 mL | 5.006 mL | 6.2575 mL |
| 50 mM | 0.0501 mL | 0.2503 mL | 0.5006 mL | 1.0012 mL | 1.2515 mL |
| 100 mM | 0.025 mL | 0.1252 mL | 0.2503 mL | 0.5006 mL | 0.6258 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
TAK-715 is a selective inhibitor of p38 MAPK with IC50 value of 7.1 nM [1].
p38 mitogen-activated protein (MAP) kinases (p38 MAPKs) are a class of mitogen-activated protein kinases and play an important role in controlling cellular responses to cytokines and stress. Four p38 MAPKs contain members, p38-α (MAPK14), p38-β (MAPK11), p38-γ (MAPK12/ERK6), and p38-δ (MAPK13/SAPK4), have been identified. Abnormal expression of p38 MAPKs are correlated with a variety of chronic inflammatory diseases and their inhibitors are regarded as promising targets in clinical [1] [2].
TAK-715 is a potent p38 MAPK inhibitor and has a different selectivity with the reported p38 MAPK inhibitor VX-745. When tested with human monocytic THP-1 cells, administration of TAK-715 exhibited inhibition on p38MAPKα with IC50 value of 7.1 nM [1]. In HEK293T, U2OS, and F9 cells, TAK-715 was used to inhibit p38 MAPK activity and concluded that p38 MAPK had no function in Wnt/β-catenin signaling pathway [2].
In adjuvant-induced rheumatoid arthritis rat model, administration of TAK-715 at dose of 10 mg/kg significantly decreased LPS-induced stimulated release of TNF-α (87.6%) by inhibiting p38 MAPK activity [1].
References:
[1]. Miwatashi, S., et al., Novel inhibitor of p38 MAP kinase as an anti-TNF-alpha drug: discovery of N-[4-[2-ethyl-4-(3-methylphenyl)-1,3-thiazol-5-yl]-2-pyridyl]benzamide (TAK-715) as a potent and orally active anti-rheumatoid arthritis agent. J Med Chem, 2005. 48(19): p. 5966-79.
[2]. Verkaar, F., et al., Inhibition of Wnt/beta-catenin signaling by p38 MAP kinase inhibitors is explained by cross-reactivity with casein kinase Idelta/varepsilon. Chem Biol, 2011. 18(4): p. 485-94.
- Picrasin B acetate
Catalog No.:BCN5215
CAS No.:30315-04-9
- Pandamarilactonine B
Catalog No.:BCN5214
CAS No.:303008-81-3
- Pandamarilactonine A
Catalog No.:BCN5213
CAS No.:303008-80-2
- Coenzyme Q10
Catalog No.:BCN5954
CAS No.:303-98-0
- Ochratoxin A
Catalog No.:BCC7008
CAS No.:303-47-9
- Gossypol
Catalog No.:BCN2702
CAS No.:303-45-7
- Methenolone enanthate
Catalog No.:BCC9029
CAS No.:303-42-4
- Lasiocarpine
Catalog No.:BCN2001
CAS No.:303-34-4
- Heliotrine
Catalog No.:BCN1982
CAS No.:303-33-3
- Clinofibrate
Catalog No.:BCC5020
CAS No.:30299-08-2
- 2-Amino-N-(2-chloro-6-methylphenyl) thiazole-5-carboxamide
Catalog No.:BCC8551
CAS No.:302964-24-5
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- Centrolobol
Catalog No.:BCN5216
CAS No.:30359-01-4
- Dalbergioidin
Catalog No.:BCN4801
CAS No.:30368-42-4
- L-779,450
Catalog No.:BCC7593
CAS No.:303727-31-3
- U 18666A
Catalog No.:BCC7136
CAS No.:3039-71-2
- Reutericyclin
Catalog No.:BCN1855
CAS No.:303957-69-9
- Hydralazine HCl
Catalog No.:BCC4911
CAS No.:304-20-1
- Harmaline
Catalog No.:BCN5218
CAS No.:304-21-2
- LU AA33810
Catalog No.:BCC7708
CAS No.:304008-29-5
- Corydalmine
Catalog No.:BCN5217
CAS No.:30413-84-4
- Pelirine
Catalog No.:BCN4077
CAS No.:30435-26-8
- Toxicarolisoflavone
Catalog No.:BCN6468
CAS No.:3044-60-8
- Dynasore
Catalog No.:BCC1088
CAS No.:304448-55-3
X-ray structure of p38alpha bound to TAK-715: comparison with three classic inhibitors.[Pubmed:22868770]
Acta Crystallogr D Biol Crystallogr. 2012 Aug;68(Pt 8):1041-50.
The p38alpha mitogen-activated protein kinase regulates the synthesis of pro-inflammatory cytokines in response to stimulation by a diverse set of stress signals. Various different chemotypes and clinical candidates that inhibit p38alpha function have been reported over the years. In this publication, the novel structure of p38alpha cocrystallized with the clinical candidate TAK-715 is reported. Owing to the impact of crystallization conditions on the conformation of protein kinases (and in particular p38alpha), the structures of complexes of p38alpha with SB-203580, SCIO-469 and VX-745 have also been determined to enable in-depth comparison of ligand-induced protein conformations. The impact of experimental conditions on p38alpha-inhibitor complex structures, most importantly soaking versus cocrystallization, is discussed. Analysis of the structures and quantification of the protein-ligand interactions couples ligand-induced protein conformations to the number of interactions and to inhibitor selectivity against the human kinome. This shows that for the design of novel kinase inhibitors, selectivity is best obtained through maximization of the number of interactions throughout the ATP pocket and the exploitation of specific features in the active site.
Novel inhibitor of p38 MAP kinase as an anti-TNF-alpha drug: discovery of N-[4-[2-ethyl-4-(3-methylphenyl)-1,3-thiazol-5-yl]-2-pyridyl]benzamide (TAK-715) as a potent and orally active anti-rheumatoid arthritis agent.[Pubmed:16162000]
J Med Chem. 2005 Sep 22;48(19):5966-79.
The p38 mitogen-activated protein (MAP) kinase has been implicated in the proinflammatory cytokine signal pathway, and its inhibitors are potentially useful for the treatment of chronic inflammatory diseases such as rheumatoid arthritis (RA) and inflammatory bowel disease. To develop a new drug for RA, we synthesized a novel series of 4-phenyl-5-pyridyl-1,3-thiazoles and evaluated their inhibition of p38 MAP kinase, lipopolysaccharide (LPS)-stimulated release of tumor necrosis factor-alpha (TNF-alpha) from human monocytic THP-1 cells in vitro, and LPS-induced TNF-alpha production in vivo in mice. During the course of the study, we found that these compounds risk the inhibition of cytochrome P450 (CYP) isoforms by coordination of the 4-pyridyl nitrogen with heme iron. We therefore investigated the effects of substitution at the 2-position of the pyridyl ring on the inhibitory activity of p38 MAP kinase and CYPs in more detail. As a result, N-[4-[2-ethyl-4-(3-methylphenyl)-1,3-thiazol-5-yl]-2-pyridyl]benzamide (8h, TAK-715) exhibited potent inhibitory activity in these assays (inhibition of p38alpha, IC50 = 7.1 nM; LPS-stimulated release of TNF-alpha from THP-1, IC50 = 48 nM; LPS-induced TNF-alpha production in mice, 87.6% inhibition at 10 mg/kg, po) and no inhibitory activity for major CYPs, including CYP3A4. This compound also showed good bioavailability in mice and rats and significant efficacy in a rat adjuvant-induced arthritis model. Compound 8h was selected as a clinical candidate and is now under clinical investigation for the treatment of RA.
Inhibition of Wnt/beta-catenin signaling by p38 MAP kinase inhibitors is explained by cross-reactivity with casein kinase Idelta/varepsilon.[Pubmed:21513885]
Chem Biol. 2011 Apr 22;18(4):485-94.
Wnt/beta-catenin signaling plays essential roles in embryonic development, adult stem cell maintenance, and disease. Screening of a small molecule compound library with a beta-galactosidase fragment complementation assay measuring beta-catenin nuclear entry revealed TAK-715 and AMG-548 as inhibitors of Wnt-3a-stimulated beta-catenin signaling. TAK-715 and AMG-548 are inhibitors of p38 mitogen-activated protein kinase, which has been suggested to regulate activation of Wnt/beta-catenin signaling. However, two highly selective and equally potent p38 inhibitors, VX-745 and Scio-469, did not inhibit Wnt-3a-stimulated beta-catenin signaling. Profiling of TAK-715 and AMG-548 against a panel of over 200 kinases revealed cross-reactivity with casein kinase Idelta and varepsilon, which are known activators of Wnt/beta-catenin signaling. Our data demonstrate that this cross-reactivity accounts for the inhibition of beta-catenin signaling by TAK-715 and AMG-548 and argue against a role of p38 in Wnt/beta-catenin signaling.


