CorydalmineCAS# 30413-84-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 30413-84-4 | SDF | Download SDF |
| PubChem ID | 161665 | Appearance | Powder |
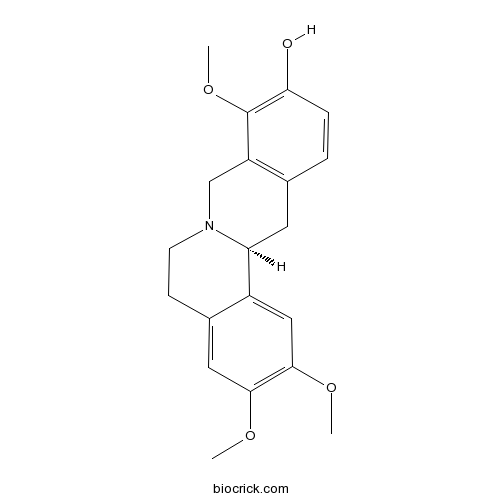
| Formula | C20H23NO4 | M.Wt | 341.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (13aS)-2,3,9-trimethoxy-6,8,13,13a-tetrahydro-5H-isoquinolino[2,1-b]isoquinolin-10-ol | ||
| SMILES | COC1=C(C=C2C3CC4=C(CN3CCC2=C1)C(=C(C=C4)O)OC)OC | ||
| Standard InChIKey | DIHXHTWYVOYYDC-INIZCTEOSA-N | ||
| Standard InChI | InChI=1S/C20H23NO4/c1-23-18-9-13-6-7-21-11-15-12(4-5-17(22)20(15)25-3)8-16(21)14(13)10-19(18)24-2/h4-5,9-10,16,22H,6-8,11H2,1-3H3/t16-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Corydalmine exhibits antibacterial activities against Staphylococcus aureus and methicillin-resistant S. aureus strains. 2. 1-Corydalmine significantly inhibits spore germination of all the fungi at 100 to 1500 ppm. 3. l-Corydalmine exhibits potent analgesic activity in preclinical models, it is under development as an oral analgesic agent. |
| Targets | P450 (e.g. CYP17) | Antifection |

Corydalmine Dilution Calculator

Corydalmine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9291 mL | 14.6456 mL | 29.2912 mL | 58.5823 mL | 73.2279 mL |
| 5 mM | 0.5858 mL | 2.9291 mL | 5.8582 mL | 11.7165 mL | 14.6456 mL |
| 10 mM | 0.2929 mL | 1.4646 mL | 2.9291 mL | 5.8582 mL | 7.3228 mL |
| 50 mM | 0.0586 mL | 0.2929 mL | 0.5858 mL | 1.1716 mL | 1.4646 mL |
| 100 mM | 0.0293 mL | 0.1465 mL | 0.2929 mL | 0.5858 mL | 0.7323 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- LU AA33810
Catalog No.:BCC7708
CAS No.:304008-29-5
- Harmaline
Catalog No.:BCN5218
CAS No.:304-21-2
- Hydralazine HCl
Catalog No.:BCC4911
CAS No.:304-20-1
- Reutericyclin
Catalog No.:BCN1855
CAS No.:303957-69-9
- U 18666A
Catalog No.:BCC7136
CAS No.:3039-71-2
- L-779,450
Catalog No.:BCC7593
CAS No.:303727-31-3
- Dalbergioidin
Catalog No.:BCN4801
CAS No.:30368-42-4
- Centrolobol
Catalog No.:BCN5216
CAS No.:30359-01-4
- TAK-715
Catalog No.:BCC3968
CAS No.:303162-79-0
- Picrasin B acetate
Catalog No.:BCN5215
CAS No.:30315-04-9
- Pandamarilactonine B
Catalog No.:BCN5214
CAS No.:303008-81-3
- Pandamarilactonine A
Catalog No.:BCN5213
CAS No.:303008-80-2
- Pelirine
Catalog No.:BCN4077
CAS No.:30435-26-8
- Toxicarolisoflavone
Catalog No.:BCN6468
CAS No.:3044-60-8
- Dynasore
Catalog No.:BCC1088
CAS No.:304448-55-3
- AVE 0991
Catalog No.:BCC4032
CAS No.:304462-19-9
- Cyclomusalenone
Catalog No.:BCN4654
CAS No.:30452-60-9
- Theaflavin-3-gallate
Catalog No.:BCN2316
CAS No.:30462-34-1
- Theaflavin 3,3'-di-O-gallate
Catalog No.:BCN5920
CAS No.:30462-35-2
- Xerophilusin G
Catalog No.:BCN5219
CAS No.:304642-94-2
- S 24795
Catalog No.:BCC7700
CAS No.:304679-75-2
- Flunarizine 2HCl
Catalog No.:BCC4398
CAS No.:30484-77-6
- Mearnsitrin
Catalog No.:BCN5220
CAS No.:30484-88-9
- Tanaproget
Catalog No.:BCC1984
CAS No.:304853-42-7
Effect of 1-corydalmine,an Alkaloid Isolated from Corydalis chaerophylla Roots on Spore Germination of Some Fungi.[Pubmed:24015073]
Mycobiology. 2007 Jun;35(2):69-71.
1-Corydalmine,an alkaloid isolated from roots of Corydalis chaerophylla inhibited spore germination of some plant pathogenic as well as saprophytic fungi e.g. Alternaria brassicae, A. brassicicola, A. solani, Curvularia lunata, C. maculans, C. sp., C. pallscens, Erysiphe pisi, Fusarium udum, Helminthosporium species,H. penniseti and a Heterosporium species. 1-Corydalmine significantly inhibited spore germination of all the fungi at 100 to 1500 ppm. It was effective against all the fungi at 1500 ppm. C. lunata was highly sensitive to this chemical even at 250 ppm.
In vitro metabolism of l-corydalmine, a potent analgesic drug, in human, cynomolgus monkey, beagle dog, rat and mouse liver microsomes.[Pubmed:27239758]
J Pharm Biomed Anal. 2016 Sep 5;128:98-105.
l-Corydalmine (l-CDL) was under development as an oral analgesic agent, exhibiting potent analgesic activity in preclinical models. The objective of this study was to compare metabolic profiles of l-CDL in liver microsomes from mouse, rat, monkey, dog and human. Six metabolites (M1-M6) were identified using LC-Q/TOF in liver microsomes from the five species. The metabolism of l-CDL included O-demethylation (M1-3) and hydroxylation (M4-6). The desmethyl metabolites were the major ones among the five species, which accounted for more than 84%. Data from chemical inhibition in human liver microsomes (HLM) and human recombinant CYP450s demonstrated that CYP2D6 exhibited strong catalytic activity towards M1 and M2 formations, while CYP2C9 and CYP2C19 also catalyzed M2 formation. Formations of M3 and hydroxyl metabolites (M4 and M5) were mainly catalyzed by CYP3A4. Further studies showed that M1 and M2 were main metabolites in HLM. The kinetics of M1 and M2 formations in HLM and recombinant CYP450s were also investigated. The results showed that M1 and M2 formations in HLM and recombinant CYP2D6 characterized biphasic kinetics, whereas sigmoid Vmax model was better used to fit M2 formation by recombinant CYP2C9 and CYP2C19. The contributions of CYP2D6 to M1 and M2 formations in HLM were estimated to be 75.3% and 50.7%, respectively. However, the contributions of CYP2C9 and CYP2C19 to M2 formation were only 5.0% and 4.1%, respectively. All these data indicated that M1 and M2 were main metabolites in HLM, and CYP2D6 was the primary enzyme responsible for their formations.
A new antibacterial denitroaristolochic acid from the tubers of Stephania succifera.[Pubmed:23418880]
J Asian Nat Prod Res. 2013;15(3):315-8.
A new denitroaristolochic acid, demethylaristofolin C (1), together with six known alkaloids, crebanine N-oxide (2), (-)-sukhodianine-beta-N-oxide (3), palmatine (4), Corydalmine (5), dehydroCorydalmine (6), and corynoxidine (7), was isolated from the tubers of Stephania succifera. The structure of demethylaristofolin C was elucidated by spectroscopic techniques (UV, IR, 1D, and 2D NMR) and HR-ESI-MS analyses. These compounds exhibited antibacterial activities against Staphylococcus aureus and methicillin-resistant S. aureus strains in different degrees.


