Dasatinib (BMS-354825)Src and BCR-Abl inhibitor CAS# 302962-49-8 |
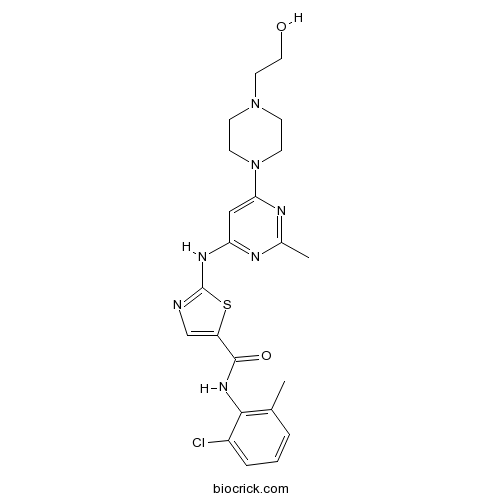
- DCC-2036 (Rebastinib)
Catalog No.:BCC4390
CAS No.:1020172-07-9
- PP 2 (AG 1879)
Catalog No.:BCC3631
CAS No.:172889-27-9
- WH-4-023
Catalog No.:BCC8051
CAS No.:837422-57-8
- TG 100572
Catalog No.:BCC1994
CAS No.:867334-05-2
- KX2-391
Catalog No.:BCC5080
CAS No.:897016-82-9
- XL228
Catalog No.:BCC2058
CAS No.:898280-07-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 302962-49-8 | SDF | Download SDF |
| PubChem ID | 3062316 | Appearance | Powder |
| Formula | C22H26ClN7O2S | M.Wt | 488.01 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BMS-354825 | ||
| Solubility | DMSO : 35.35 mg/mL (72.44 mM; Need ultrasonic and warming) | ||
| Chemical Name | N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-yl]amino]-1,3-thiazole-5-carboxamide | ||
| SMILES | CC1=C(C(=CC=C1)Cl)NC(=O)C2=CN=C(S2)NC3=NC(=NC(=C3)N4CCN(CC4)CCO)C | ||
| Standard InChIKey | ZBNZXTGUTAYRHI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Targets | Abl | Src | c-Kit (WT)/c-Kit (D816V) | |||
| IC50 | 0.6 nM | 0.8 nM | 79 nM/37 nM |
| Cell experiment: [1] | |
| Cell lines | DU-145 and LNCaP cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 100 nM, 6 hours for inhibiting FAK phosphorylation 24 hours for decreasing cell-to-cell contact |
| Applications | Dasatinib almost totally abolished the levels of p-FAK at Tyr576/577 in DU-145 cells, whereas p-FAK was not detected in LNCaP cells even though both cell lines expressed similar levels of total FAK protein. Treatment with 100 nmol/L dasatinib for 24 hours had no effect on cell viability and total cell numbers, although partial inhibition of cell proliferation due to G1 arrest was observed at 48 and 72 hours. Besides, there was a substantial loss of cell-to-cell contact in DU-145 cells. This effect may be related to the decrease in levels of p-FAK and p-p130CAS. |
| Animal experiment: [2] | |
| Animal models | Pdx1-Cre, Z/EGFP, LSL-Kras G12D/+, LSL-Trp53R172H/+ mice |
| Dosage form | Oral administration, 10 mg/kg, daily |
| Application | There was no significant difference in survival between the different treatment groups. The median survival of vehicle-treated animals was 131 days compared with 127 days and 130 days for animals treated with dasatinib from 6 weeks and 10 weeks of age, respectively. Analysis of tumor burden in the mice showed that all mice had invasive PDAC; however, the number of mice with metastases was reduced significantly in dasatinib-treated animals. The incidence of metastases was 61.1% in vehicle-treated animals compared with 26.7% in mice treated with dasatinib from 6 weeks and 23.1% in mice treated with dasatinib from 10 weeks. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Nam S, Kim D, Cheng J Q, et al. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer research, 2005, 65(20): 9185-9189. [2] Morton J P, Karim S A, Graham K, et al. Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology, 2010, 139(1): 292-303. | |

Dasatinib (BMS-354825) Dilution Calculator

Dasatinib (BMS-354825) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0491 mL | 10.2457 mL | 20.4914 mL | 40.9828 mL | 51.2285 mL |
| 5 mM | 0.4098 mL | 2.0491 mL | 4.0983 mL | 8.1966 mL | 10.2457 mL |
| 10 mM | 0.2049 mL | 1.0246 mL | 2.0491 mL | 4.0983 mL | 5.1228 mL |
| 50 mM | 0.041 mL | 0.2049 mL | 0.4098 mL | 0.8197 mL | 1.0246 mL |
| 100 mM | 0.0205 mL | 0.1025 mL | 0.2049 mL | 0.4098 mL | 0.5123 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
Randomized trials have been conducted to compare the effects of dasatinib, nilotinib and imatinib in the treatment of newly diagnosed chronic myrloid leukemia in the chronic phase.
Abstract
Dasatinib is an inhibitor of tyrosine kinase including Src kinases that exhibits antitumor activity, affects osteoclasts and synergize with docetaxel. The efficacy of dasatinib alone or with docetaxel has been assessed in chemotherapy-naive men with metastatic castration-resistant prostate cancer.
Abstract
Dasatinib is a dual Abl/Src TKI that inhibits BCR-ABL with relatively greater potency and show potential immunomodulatory effects. Since it’s been approved to treat CML patients, the development of dasatinib is reviewed.
Abstract
Daily oral administration of dasatinib, an inhibitor of Src family kinases, to mice with breast cancer delays tumor onset and increases overall survival with the occurrence of squamous metaplasis accompanied by down-regulation of ErbB-2 and up-regulation of E-cadherin and β-catenin. Additionally, dasatinib inhibited both migration and invasion of tumour-derived cell lines in vitro.
Abstract
Dasatinib is an inhibitor of multiple tyrosine kinases that are used to treat CML and ALL. Although renal failure is a rare side effect of dasatinib, a patient with imatinib-resistant CML has developed both PE and ARF after receiving dastinib.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Dasatinib is a small-molecule inhibitor of both the Src and Bcr-Abl tyrosine kinases with IC50 values of 0.5 nM and 1 nM. The oncogenic tyrosine kinase Bcr-Abl, plays a critical role in the pathogenesis of CML (chronic myelogenous leukemia). In most CML patients, the kinase domain of Bcr-Abl have mutations that interfere with binding to the first Abl kinase inhibitor, but Dasatinib bind more efficiently to these Abl kinase mutations and thus more effective than imatinib in inhibiting the proliferation cells with wild-type Bcr-Abl or Bcr-Abl mutants.
References
1. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. S Nam, D Kim, JQ Cheng, S Zhang, JH Lee, R Buettner. Cancer Research. 2005
2. Dasatinib (BMS-354825) selectively induces apoptosis in lung cancer cells dependent on epidermal growth factor receptor signaling for survival. L Song, M Morris, T Bagui, FY Lee, R Jove, EB Haura . Cancer Research. 2006
- Ro 67-4853
Catalog No.:BCC7921
CAS No.:302841-89-0
- Ro 01-6128
Catalog No.:BCC7922
CAS No.:302841-86-7
- Ciliobrevin A
Catalog No.:BCC3939
CAS No.:302803-72-1
- TCS 46b
Catalog No.:BCC7482
CAS No.:302799-86-6
- 3,4,5-Trimethoxycinnamyl alcohol
Catalog No.:BCN5212
CAS No.:30273-62-2
- Bisline
Catalog No.:BCN2062
CAS No.:30258-28-7
- Rosiglitazone HCl
Catalog No.:BCC2269
CAS No.:302543-62-0
- Potassium 7-hydroxynaphthalene-1-sulfonate
Catalog No.:BCN8289
CAS No.:30252-40-5
- 1-Hydroxybaccatin I
Catalog No.:BCN5211
CAS No.:30244-37-2
- Ingenol
Catalog No.:BCN2333
CAS No.:30220-46-3
- Effusanin A
Catalog No.:BCN5210
CAS No.:30220-43-0
- Arenobufagin 3-hemisuberate
Catalog No.:BCN7837
CAS No.:30219-16-0
- 2-Amino-N-(2-chloro-6-methylphenyl) thiazole-5-carboxamide
Catalog No.:BCC8551
CAS No.:302964-24-5
- Clinofibrate
Catalog No.:BCC5020
CAS No.:30299-08-2
- Heliotrine
Catalog No.:BCN1982
CAS No.:303-33-3
- Lasiocarpine
Catalog No.:BCN2001
CAS No.:303-34-4
- Methenolone enanthate
Catalog No.:BCC9029
CAS No.:303-42-4
- Gossypol
Catalog No.:BCN2702
CAS No.:303-45-7
- Ochratoxin A
Catalog No.:BCC7008
CAS No.:303-47-9
- Coenzyme Q10
Catalog No.:BCN5954
CAS No.:303-98-0
- Pandamarilactonine A
Catalog No.:BCN5213
CAS No.:303008-80-2
- Pandamarilactonine B
Catalog No.:BCN5214
CAS No.:303008-81-3
- Picrasin B acetate
Catalog No.:BCN5215
CAS No.:30315-04-9
- TAK-715
Catalog No.:BCC3968
CAS No.:303162-79-0
The Src/ABL kinase inhibitor dasatinib (BMS-354825) inhibits function of normal human T-lymphocytes in vitro.[Pubmed:18395492]
Clin Immunol. 2008 Jun;127(3):330-9.
Dasatinib (BMS-354825) is a Src/ABL tyrosine kinase inhibitor currently approved for the treatment of chronic myeloid leukemia. Dasatinib has increased potency against ABL compared to the current therapy imatinib, and is effective in many cases where disease is resistant to imatinib. Dasatinib also inhibits many Src-family tyrosine kinases. We have demonstrated in this study that dasatinib is able to block the function of normal human T-lymphocytes in vitro at clinically relevant concentrations. T-cell functions including proliferation, activation and cytokine production were all uniformly inhibited in the presence of dasatinib. We also demonstrated inhibition of TCR signalling through Src-family kinase LCK, and predicted that inhibition of LCK and other kinases involved in T-cell signalling by dasatinib is responsible for the suppression of T-cell function. These findings raise the concern about potential T-cell inhibition in patients taking dasatinib, and suggest a possible application for the treatment of T-cell mediated immune disorders.
Phase II study of dasatinib (BMS-354825) in patients with metastatic adenocarcinoma of the pancreas.[Pubmed:24072218]
Oncologist. 2013;18(10):1091-2.
BACKGROUND: Src, EphA2, and platelet-derived growth factor receptors alpha and beta are dysregulated in pancreatic ductal adenocarcinoma (PDAC). METHODS: Dasatinib is an oral multitarget tyrosine kinase inhibitor that targets BCR-ABL, c-Src, c-KIT, platelet-derived growth factor receptor beta, and EphA2. We conducted a phase II, single-arm study of dasatinib as first-line therapy in patients with metastatic PDAC. METHODS: Dasatinib (100 mg twice a day, later reduced to 70 mg twice a day because of toxicities) was orally administered continuously on a 28-day cycle. The primary endpoint was overall survival (OS). Response was measured using the Response Evaluation Criteria in Solid Tumors. Circulating tumor cells (CTCs) were also collected. RESULTS: Fifty-one patients enrolled in this study. The median OS was 4.7 months (95% confidence interval [CI]: 2.8-6.9 months). Median progression-free survival was 2.1 months (95% CI: 1.6-3.2 months). In 34 evaluable patients, the best response achieved was stable disease in 10 patients (29.4%). One patient had stable disease while on treatment for 20 months. The most common nonhematologic toxicities were fatigue and nausea. Edema and pleural effusions occurred in 29% and 6% of patients, respectively. The number of CTCs did not correlate with survival. CONCLUSION: Single-agent dasatinib does not have clinical activity in metastatic PDAC.
Phase I study of dasatinib (BMS-354825) in Japanese patients with solid tumors.[Pubmed:21781226]
Cancer Sci. 2011 Nov;102(11):2058-64.
Dasatinib is a potent oral inhibitor of tyrosine kinases including the SRC family kinases, which are activated in tumors, and implicated in invasion and bone metastasis. This phase I dose-escalation study assessed safety, tolerability, maximum tolerated dose (MTD), antitumor activity, pharmacokinetics and pharmacodynamics in Japanese patients with refractory, advanced solid tumors. Dasatinib was administered once daily at 100, 150 and 200 mg/day. Sixteen patients were treated with dasatinib in the following doses: 100 mg (nine patients), 150 mg (three patients) and 200 mg (four patients). The most frequent adverse events (AE; >/= 50%) were anorexia, fatigue, pleural effusion, anemia, constipation, diarrhea, vomiting and increased aspartate aminotransferase (AST). The most frequent AE of grade >/= 3 (>/= 10%) were anemia, decreased lymphocyte count, fatigue and increased blood magnesium. Dose-limiting toxicities were observed in two patients: grade 2 pleural effusion and bronchial wall thickening at the 100-mg level and grade 3 dyspnea at the 200-mg level. In addition, grade 2 pleural effusion was observed in all four patients treated with 200 mg. Therefore, 150 mg was determined to be the MTD. The pharmacokinetic parameters were comparable among the dose levels. As a pharmacodynamic study, markers of bone metabolism were assessed. Bone resorption markers, NTx and TRACP-5b, showed a decrease of 46.3% and 22.2%, respectively. No objective responses were observed, but three patients had stable disease that lasted for over 6 months. In this study population, the safety profile of dasatinib was generally acceptable and 150 mg of dasatinib administered once daily was determined to be the MTD.
Molecular mechanisms of action and potential biomarkers of growth inhibition of dasatinib (BMS-354825) on hepatocellular carcinoma cells.[Pubmed:23721490]
BMC Cancer. 2013 May 30;13:267.
BACKGROUND: Molecular targeted therapy has emerged as a promising treatment of Hepatocellular carcinoma (HCC). One potential target is the Src family Kinase (SFK). C-Src, a non-receptor tyrosine kinase is a critical link of multiple signal pathways that regulate proliferation, invasion, survival, metastasis, and angiogenesis. In this study, we evaluated the effects of a novel SFK inhibitor, Dasatinib (BMS-354825), on SFK/FAK/p130CAS, PI3K/PTEN/Akt/mTOR, Ras/Raf/MAPK and Stats pathways in 9 HCC cell lines. METHODS: Growth inhibition was assessed by MTS assay. EGFR, Src and downstream proteins FAK, Akt, MAPK42/44, Stat3 expressions were measured by western blot. Cell adhesion, migration and invasion were performed with and without dasatinib treatment. RESULTS: The IC50 of 9 cell lines ranged from 0.7 muM ~ 14.2 muM. In general the growth inhibition by dasatinib was related to total Src (t-Src) and the ratio of activated Src (p-Src) to t-Src. There was good correlation of the sensitivity to dasatinib and the inhibition level of p-Src, p-FAK576/577 and p-Akt. No inhibition was found on Stat3 and MAPK42/44 in all cell lines. The inhibition of cell adhesion, migration and invasion were correlated with p-FAK inhibition. CONCLUSION: Dasatinib inhibits the proliferation, adhesion, migration and invasion of HCC cells in vitro via inhibiting of Src tyrosine kinase and affecting SFK/FAK and PI3K/PTEN/Akt, but not Ras/Raf/MEK/ERK and JAK/Stat pathways. T-Src and p-Src/t-Src may be useful biomarkers to select HCC patients for dasatinib treatment.


