Ciliobrevin AHh pathway antagonist CAS# 302803-72-1 |
- Cyclopamine
Catalog No.:BCN2964
CAS No.:4449-51-8
- Purmorphamine
Catalog No.:BCC3641
CAS No.:483367-10-8
- GANT61
Catalog No.:BCC1090
CAS No.:500579-04-4
- GANT 58
Catalog No.:BCC1587
CAS No.:64048-12-0
- GDC-0449 (Vismodegib)
Catalog No.:BCC1285
CAS No.:879085-55-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 302803-72-1 | SDF | Download SDF |
| PubChem ID | 6883982 | Appearance | Powder |
| Formula | C17H9Cl2N3O2 | M.Wt | 358.18 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | HPI-4 | ||
| Solubility | DMSO : 100 mg/mL (279.19 mM; Need ultrasonic) | ||
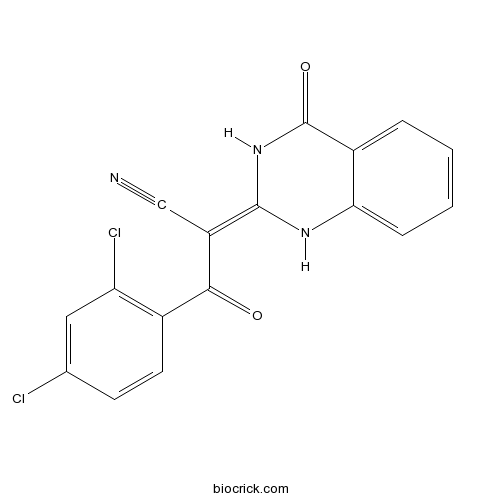
| Chemical Name | (2E)-3-(2,4-dichlorophenyl)-3-oxo-2-(4-oxo-1H-quinazolin-2-ylidene)propanenitrile | ||
| SMILES | C1=CC=C2C(=C1)C(=O)NC(=C(C#N)C(=O)C3=C(C=C(C=C3)Cl)Cl)N2 | ||
| Standard InChIKey | SESYPWCSIZUIAS-FOWTUZBSSA-N | ||
| Standard InChI | InChI=1S/C17H9Cl2N3O2/c18-9-5-6-10(13(19)7-9)15(23)12(8-20)16-21-14-4-2-1-3-11(14)17(24)22-16/h1-7,21H,(H,22,24)/b16-12+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Hedgehog (Hh) pathway antagonist; blocks Sonic hedgehog (Shh)-induced Hh pathway activation (IC50 = 7 μM) downstream of Smo. Perturbs primary cilia formation; inhibits cytoplasmic AAA+ ATPase dynein-dependent microtubule gliding and ATPase activity. |

Ciliobrevin A Dilution Calculator

Ciliobrevin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7919 mL | 13.9595 mL | 27.9189 mL | 55.8378 mL | 69.7973 mL |
| 5 mM | 0.5584 mL | 2.7919 mL | 5.5838 mL | 11.1676 mL | 13.9595 mL |
| 10 mM | 0.2792 mL | 1.3959 mL | 2.7919 mL | 5.5838 mL | 6.9797 mL |
| 50 mM | 0.0558 mL | 0.2792 mL | 0.5584 mL | 1.1168 mL | 1.3959 mL |
| 100 mM | 0.0279 mL | 0.1396 mL | 0.2792 mL | 0.5584 mL | 0.698 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Hedgehog (Hh) pathway antagonist; blocks Sonic hedgehog (Shh)-induced Hh pathway activation (IC50 = 7 μM) downstream of Smo. Peturbs primary cilia formation; inhibits cytoplasmic AAA+ ATPase dynein-dependent microtubule gliding and ATPase activity.
- TCS 46b
Catalog No.:BCC7482
CAS No.:302799-86-6
- 3,4,5-Trimethoxycinnamyl alcohol
Catalog No.:BCN5212
CAS No.:30273-62-2
- Bisline
Catalog No.:BCN2062
CAS No.:30258-28-7
- Rosiglitazone HCl
Catalog No.:BCC2269
CAS No.:302543-62-0
- Potassium 7-hydroxynaphthalene-1-sulfonate
Catalog No.:BCN8289
CAS No.:30252-40-5
- 1-Hydroxybaccatin I
Catalog No.:BCN5211
CAS No.:30244-37-2
- Ingenol
Catalog No.:BCN2333
CAS No.:30220-46-3
- Effusanin A
Catalog No.:BCN5210
CAS No.:30220-43-0
- Arenobufagin 3-hemisuberate
Catalog No.:BCN7837
CAS No.:30219-16-0
- Retinoic acid
Catalog No.:BCN2185
CAS No.:302-79-4
- DL-Alanine
Catalog No.:BCN8539
CAS No.:302-72-7
- Aconitine
Catalog No.:BCN1014
CAS No.:302-27-2
- Ro 01-6128
Catalog No.:BCC7922
CAS No.:302841-86-7
- Ro 67-4853
Catalog No.:BCC7921
CAS No.:302841-89-0
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- 2-Amino-N-(2-chloro-6-methylphenyl) thiazole-5-carboxamide
Catalog No.:BCC8551
CAS No.:302964-24-5
- Clinofibrate
Catalog No.:BCC5020
CAS No.:30299-08-2
- Heliotrine
Catalog No.:BCN1982
CAS No.:303-33-3
- Lasiocarpine
Catalog No.:BCN2001
CAS No.:303-34-4
- Methenolone enanthate
Catalog No.:BCC9029
CAS No.:303-42-4
- Gossypol
Catalog No.:BCN2702
CAS No.:303-45-7
- Ochratoxin A
Catalog No.:BCC7008
CAS No.:303-47-9
- Coenzyme Q10
Catalog No.:BCN5954
CAS No.:303-98-0
- Pandamarilactonine A
Catalog No.:BCN5213
CAS No.:303008-80-2
The dynein inhibitor Ciliobrevin D inhibits the bidirectional transport of organelles along sensory axons and impairs NGF-mediated regulation of growth cones and axon branches.[Pubmed:25404503]
Dev Neurobiol. 2015 Jul;75(7):757-77.
The axonal transport of organelles is critical for the development, maintenance, and survival of neurons, and its dysfunction has been implicated in several neurodegenerative diseases. Retrograde axon transport is mediated by the motor protein dynein. In this study, using embryonic chicken dorsal root ganglion neurons, we investigate the effects of Ciliobrevin D, a pharmacological dynein inhibitor, on the transport of axonal organelles, axon extension, nerve growth factor (NGF)-induced branching and growth cone expansion, and axon thinning in response to actin filament depolymerization. Live imaging of mitochondria, lysosomes, and Golgi-derived vesicles in axons revealed that both the retrograde and anterograde transport of these organelles was inhibited by treatment with Ciliobrevin D. Treatment with Ciliobrevin D reversibly inhibits axon extension and transport, with effects detectable within the first 20 min of treatment. NGF induces growth cone expansion, axonal filopodia formation and branching. Ciliobrevin D prevented NGF-induced formation of axonal filopodia and branching but not growth cone expansion. Finally, we report that the retrograde reorganization of the axonal cytoplasm which occurs on actin filament depolymerization is inhibited by treatment with Ciliobrevin D, indicating a role for microtubule based transport in this process, as well as Ciliobrevin D accelerating Wallerian degeneration. This study identifies Ciliobrevin D as an inhibitor of the bidirectional transport of multiple axonal organelles, indicating this drug may be a valuable tool for both the study of dynein function and a first pass analysis of the role of axonal transport.
Effects of the dynein inhibitor ciliobrevin on the flagellar motility of sea urchin spermatozoa.[Pubmed:25809136]
Cytoskeleton (Hoboken). 2015 Apr;72(4):182-92.
Ciliobrevin has recently been found to be a membrane-permeable inhibitor that is specific to AAA+ molecular motors such as cytoplasmic dyneins. In this study, we investigated how ciliobrevin inhibited the motility of sperm from sea urchins: Hemicentrotus pulcherrimus, Pseudocentrotus depressus, and Anthocidaris crassispina. After application of 100 muM of Ciliobrevin A to live spermatozoa, swimming speed decreased gradually and flagellar motion stopped almost completely within 5 to 10 min. This inhibition was reversible and the frequency of flagellar beating was reduced in a concentration-dependent manner. Ciliobrevin had similar inhibitory effects on the flagellar beating of demembranated and reactivated sperm and the sliding disintegration of trypsin-treated axonemes. We also analyzed the curvature and shear angle of the beating flagella and found that the proximal region of the sperm flagellum was less sensitive to ciliobrevin compared with more distal regions, where bending motions were blocked completely. Interestingly, the shear angle analysis of flagellar motility showed that ciliobrevin induced highly asymmetric bends in the proximal region of the flagellum. These results suggest that there is heterogeneity in the inhibitory thresholds of dynein motors, which depend on the regions along the flagellar shaft (proximal or distal) and on the sites of doublets in the flagellar cross-section (doublet numbers). We expect that it will be possible to map the functional differences in dynein subtypes along and/or around the cross-sections of flagellar axonemes by analyzing the inhibitory effects of ciliobrevin.
Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein.[Pubmed:22425997]
Nature. 2012 Mar 18;484(7392):125-9.
The conversion of chemical energy into mechanical force by AAA+ (ATPases associated with diverse cellular activities) ATPases is integral to cellular processes, including DNA replication, protein unfolding, cargo transport and membrane fusion. The AAA+ ATPase motor cytoplasmic dynein regulates ciliary trafficking, mitotic spindle formation and organelle transport, and dissecting its precise functions has been challenging because of its rapid timescale of action and the lack of cell-permeable, chemical modulators. Here we describe the discovery of ciliobrevins, the first specific small-molecule antagonists of cytoplasmic dynein. Ciliobrevins perturb protein trafficking within the primary cilium, leading to their malformation and Hedgehog signalling blockade. Ciliobrevins also prevent spindle pole focusing, kinetochore-microtubule attachment, melanosome aggregation and peroxisome motility in cultured cells. We further demonstrate the ability of ciliobrevins to block dynein-dependent microtubule gliding and ATPase activity in vitro. Ciliobrevins therefore will be useful reagents for studying cellular processes that require this microtubule motor and may guide the development of additional AAA+ ATPase superfamily inhibitors.
Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade.[Pubmed:19666565]
Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):14132-7.
Inappropriate activation of the Hedgehog (Hh) signaling pathway has been implicated in a diverse spectrum of cancers, and its pharmacological blockade has emerged as an anti-tumor strategy. While nearly all known Hh pathway antagonists target the transmembrane protein Smoothened (Smo), small molecules that suppress downstream effectors could more comprehensively remediate Hh pathway-dependent tumors. We report here four Hh pathway antagonists that are epistatic to the nucleocytoplasmic regulator Suppressor of Fused [Su(fu)], including two that can inhibit Hh target gene expression induced by overexpression of the Gli transcription factors. Each inhibitor has a unique mechanism of action, and their phenotypes reveal that Gli processing, Gli activation, and primary cilia formation are pharmacologically targetable. We further establish the ability of certain compounds to block the proliferation of cerebellar granule neuron precursors expressing an oncogenic form of Smo, and we demonstrate that Hh pathway inhibitors can have tissue-specific activities. These antagonists therefore constitute a valuable set of chemical tools for interrogating downstream Hh signaling mechanisms and for developing chemotherapies against Hh pathway-related cancers.


