AconitineCAS# 302-27-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 302-27-2 | SDF | Download SDF |
| PubChem ID | 245005 | Appearance | White powder |
| Formula | C34H47NO11 | M.Wt | 645.75 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in ethanol; sparingly soluble in water | ||
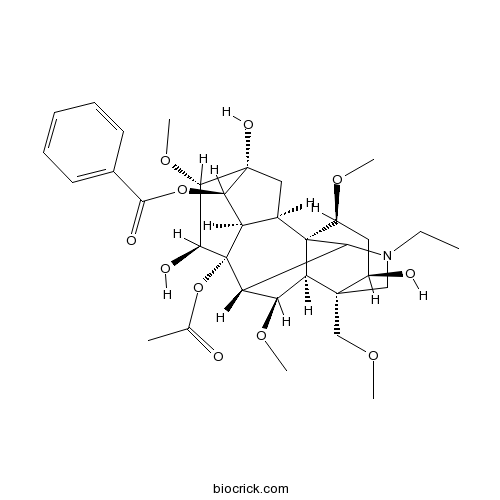
| SMILES | CCN1CC2(C(CC(C34C2C(C(C31)C5(C6C4CC(C6OC(=O)C7=CC=CC=C7)(C(C5O)OC)O)OC(=O)C)OC)OC)O)COC | ||
| Standard InChIKey | XFSBVAOIAHNAPC-XTHSEXKGSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Aconitine, one of the major Aconitum alkaloids, is a highly toxic compound from the Aconitum species, it appears to exert a long-lasting cholinergic action in the causation of severe arrhythmias leading to death. |
| Targets | P450 (e.g. CYP17) |
| In vivo | Effects of long-term administrations of aconitine on electrocardiogram and tissue concentrations of aconitine and its metabolites in mice.[Pubmed: 15607586 ]Forensic Sci Int. 2005 Feb 10;148(1):21-9.Aconitum alkaloids are well known for their acute and high toxicity, for example, in the causation of severe arrhythmias leading to death. Aconitine, one of the major Aconitum alkaloids, is a highly toxic compound from the Aconitum species. However, there has been no studies reported on the influence of the chronic administration of Aconitine.
Thus, this study was conducted to investigate the influence of chronic administration of Aconitine in experimental animal models. |
| Kinase Assay | The influences of aconitine, an active/toxic alkaloid from aconitum, on the oral pharmacokinetics of CYP3A probe drug buspirone in rats.[Pubmed: 25434398]Drug Metab Lett. 2014;8(2):135-44.Aconitine (AC), an active/toxic alkaloid from Aconitum species, is commonly present in Traditional Chinese Medicine (TCM) prescriptions because of the great effectiveness of Aconitum for the treatment of rheumatoid arthritis, cardiovascular diseases, and tumors in clinic. Buspirone (BP) is a sensitive CYP3A probe drug that is administered through oral/intravenous routes as recommended by the U.S. Food and Drug Administration.
|
| Animal Research | Hemodynamic and arrhythmogenic effects of aconitine applied to the left atria of anesthetized cats. Effects of amiodarone and atropine.[Pubmed: 6160357]J Cardiovasc Pharmacol. 1981 Jan-Feb;3(1):87-100.
|
| Structure Identification | J Pharm Sci. 2014 Nov;103(11):3602-10.An in vitro and in vivo comparison of solid and liquid-oil cores in transdermal aconitine nanocarriers.[Pubmed: 25187419]
Zhong Yao Cai. 2014 Feb;37(2):284-7.[Pharmacokinetic study of six aconitine alkaloids in aconiti lateralis radix praeparata in beagle dogs].[Pubmed: 25095352]To study the pharmacokinetics characteristics of six Aconitum alkaloids Aconitine (AC), mesAconitine (MA), hypAconitine (HA), benzoylaconine (BAC), benzoylmesaconine (BMA) and benzoylhypaconine (BHA) in beagle dogs.
|

Aconitine Dilution Calculator

Aconitine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5486 mL | 7.7429 mL | 15.4859 mL | 30.9717 mL | 38.7147 mL |
| 5 mM | 0.3097 mL | 1.5486 mL | 3.0972 mL | 6.1943 mL | 7.7429 mL |
| 10 mM | 0.1549 mL | 0.7743 mL | 1.5486 mL | 3.0972 mL | 3.8715 mL |
| 50 mM | 0.031 mL | 0.1549 mL | 0.3097 mL | 0.6194 mL | 0.7743 mL |
| 100 mM | 0.0155 mL | 0.0774 mL | 0.1549 mL | 0.3097 mL | 0.3871 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hydroxyprogesterone acetate
Catalog No.:BCC8997
CAS No.:302-23-8
- Desoxyrhaponticin
Catalog No.:BCN2954
CAS No.:30197-14-9
- Ro 5-3335
Catalog No.:BCC7962
CAS No.:30195-30-3
- Boc-Lys(Boc)-OSu
Catalog No.:BCC3415
CAS No.:30189-36-7
- D4476
Catalog No.:BCC1508
CAS No.:301836-43-1
- SB 431542
Catalog No.:BCC3658
CAS No.:301836-41-9
- Jasminin
Catalog No.:BCN7468
CAS No.:30164-93-3
- Compstatin control peptide
Catalog No.:BCC6067
CAS No.:301544-78-5
- Seneganolide
Catalog No.:BCN5209
CAS No.:301530-12-1
- H-Val-NH2.HCl
Catalog No.:BCC3144
CAS No.:3014-80-0
- P7C3
Catalog No.:BCC6524
CAS No.:301353-96-8
- CH 223191
Catalog No.:BCC3896
CAS No.:301326-22-7
- DL-Alanine
Catalog No.:BCN8539
CAS No.:302-72-7
- Retinoic acid
Catalog No.:BCN2185
CAS No.:302-79-4
- Arenobufagin 3-hemisuberate
Catalog No.:BCN7837
CAS No.:30219-16-0
- Effusanin A
Catalog No.:BCN5210
CAS No.:30220-43-0
- Ingenol
Catalog No.:BCN2333
CAS No.:30220-46-3
- 1-Hydroxybaccatin I
Catalog No.:BCN5211
CAS No.:30244-37-2
- Potassium 7-hydroxynaphthalene-1-sulfonate
Catalog No.:BCN8289
CAS No.:30252-40-5
- Rosiglitazone HCl
Catalog No.:BCC2269
CAS No.:302543-62-0
- Bisline
Catalog No.:BCN2062
CAS No.:30258-28-7
- 3,4,5-Trimethoxycinnamyl alcohol
Catalog No.:BCN5212
CAS No.:30273-62-2
- TCS 46b
Catalog No.:BCC7482
CAS No.:302799-86-6
- Ciliobrevin A
Catalog No.:BCC3939
CAS No.:302803-72-1
[Pharmacokinetic study of six aconitine alkaloids in aconiti lateralis radix praeparata in beagle dogs].[Pubmed:25095352]
Zhong Yao Cai. 2014 Feb;37(2):284-7.
OBJECTIVE: To study the pharmacokinetics characteristics of six Aconitum alkaloids Aconitine (AC), mesAconitine (MA), hypAconitine (HA), benzoylaconine (BAC), benzoylmesaconine (BMA) and benzoylhypaconine (BHA) in beagle dogs. METHODS: An ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method was developed for simultaneous quantitation of six Aconitum alkaloids in beagle dog plasma after oral administration of Aconiti Lateralis Radix Praeparata decoction. UPLC/MS/MS system coupled with an electrospray ionization (ESI) source was performed in multiple-reaction monitoring (MRM) mode. Sample preparation was performed with solid-phase extraction(SPE) on a 3 mL HLB cartridge before the analysis. The separation was applied on a Waters C8 column (100 mm x 2.1 mm, 1.7 microm) and a gradient elution of methanol and 0.2% formic acid-water was used as mobile phase. The pharmacokinetic parameters were calculated by the results of the analysis through the DAS 2. 1 software (Drug and Statistics for Windows). RESULTS: The results showed that the fitting model for the six Aconitum alkaloids was the one-compartment model pharmacokinetics. CONCLUSION: The method is successfully used for the pharmacokinetic evaluation of the six Aconitum alkaloids in beagle dog plasma, it can help monitor the ADME/Tox process when taking Aconiti Lateralis Radix Praeparata by observing the pharmacokinetic process. The results provide a good reference for clinical treatment and safe application of Aconiti Lateralis Radix Praeparata.
Hemodynamic and arrhythmogenic effects of aconitine applied to the left atria of anesthetized cats. Effects of amiodarone and atropine.[Pubmed:6160357]
J Cardiovasc Pharmacol. 1981 Jan-Feb;3(1):87-100.
The arrhythmogenic and hemodynamic effects of 0.04% Aconitine solution applied locally to the left atria of cats and the effects of amiodarone (10 mg/kg) and atropine (0.2 mg/kg) on the responses to Aconitine were investigated. Cats were anesthetized with sodium pentobarbital. The left atrial and limb lead electrocardiograms and the arterial pressure were recorded. Cardiac output was determined with the thermo dilution technique. Aconitine nitrate induced dysrhythmias lasting for a mean of 111 min. Atrial fibrillation occurred in 47% of the animals. A long-lasting (at least 3 hr) cardiodepressant action was seen in response to Aconitine. Pretreatment with atropine largely prevented the hemodynamic effects of Aconitine and prevented fibrillation. However, mean maximum atrial rates recorded during successive 5 min intervals were similar in control and atropine-treated animals. Amiodarone suppressed dysrhythmias in both atropine-treated and nontreated animals at a dose which exerted only minimal cardiodepression. The method described for evaluating potential activity against supraventricular dysrhythmias in the cat is relatively simple, reproducible, and suitable for statistical analyses. Aconitine appears to exert a long-lasting cholinergic action which may be involved in the genesis of Aconitine-induced atrial fibrillation.
An in vitro and in vivo comparison of solid and liquid-oil cores in transdermal aconitine nanocarriers.[Pubmed:25187419]
J Pharm Sci. 2014 Nov;103(11):3602-3610.
This study compared transdermal Aconitine delivery using solid lipid nanoparticles (SLN) and microemulsion (ME) vehicles. Aconitine-loaded SLN and ME were formulated with the same surfactant, cosurfactant, and water content, with an equal amount of oil matrix (ATO 888 for SLN and ethyl oleate for ME). These nanosized formulations (70-90 nm) showed suitable pH values and satisfactory skin tissue biocompatibility. SLN contained a higher concentration of smaller nanoparticles, compared with that in ME. Neither of the nanocarriers penetrated across excised skin in their intact form. In vitro transdermal delivery studies found that transdermal Aconitine flux was lower from SLN than from ME (p < 0.05), but skin Aconitine deposition was higher using SLN (p < 0.05). Fluorescence-activated cell sorting indicated that in vitro uptake of fluorescently labeled SLN by human immortalized keratinocyte (HaCaT) cells was greater than that of ME, indicating that a transcellular pathway may contribute to cutaneous drug absorption more effectively from SLN. In vivo studies found that these formulations could loosen stratum corneum layers and increase skin surface crannies, which may also enhance transdermal Aconitine delivery. SLN produced a more sustained Aconitine release, indicating that compared with ME, this transdermal delivery vehicle may reduce the toxicity of this drug.
The influences of aconitine, an active/toxic alkaloid from aconitum, on the oral pharmacokinetics of CYP3A probe drug buspirone in rats.[Pubmed:25434398]
Drug Metab Lett. 2014;8(2):135-44.
Aconitine (AC), an active/toxic alkaloid from Aconitum species, is commonly present in Traditional Chinese Medicine (TCM) prescriptions because of the great effectiveness of Aconitum for the treatment of rheumatoid arthritis, cardiovascular diseases, and tumors in clinic. Buspirone (BP) is a sensitive CYP3A probe drug that is administered through oral/intravenous routes as recommended by the U.S. Food and Drug Administration. This study aims to investigate the influences of AC (0.125 mg/kg, oral) on first-pass (intestinal and hepatic) CYP3A activity by using oral BP as the probe in rats. The pharmacokinetics of oral buspirone hydrochloride at different doses (12.5, 25, and 50 mg/kg) were conducted. The pharmacokinetics of oral BP in rats pretreated with single dose or multiple doses (7-day) of AC were investigated. The plasma concentrations of BP and its major metabolites [1-(2-pyrimidinyl)piperazine (1-PP) and 6'-hydroxybuspirone (6'-OH-BP)] were determined. The formation ratios of 1-PP and 6'-OH-BP from BP (AUC0-infinity of 1-PP/AUC0-infinity of BP and AUC0-infinity of 6'-OH-BP/AUC0-infinity of BP values) showed no alternation when the dose of BP changed. Single dose of AC decreased the AUC0-infinity of BP by 53% but increased the formation ratio of 6'-OH-BP by 74% (P<0.05). Multiple AC exposure increased the AUC0-infinity of BP by 110%, and the formation ratios of 1-PP and 6'-OH-BP from BP were increased by 229% and decreased by 95%, respectively (P<0.05). Conclusively, single/multiple AC exposure did not alter the first-pass CYP3A activity when using oral BP as probe in rats. Nevertheless, multiple AC exposure had markedly changed the production of BP metabolites.
Effects of long-term administrations of aconitine on electrocardiogram and tissue concentrations of aconitine and its metabolites in mice.[Pubmed:15607586]
Forensic Sci Int. 2005 Feb 10;148(1):21-9.
Aconitum alkaloids are well known for their acute and high toxicity, for example, in the causation of severe arrhythmias leading to death. Aconitine, one of the major Aconitum alkaloids, is a highly toxic compound from the Aconitum species. However, there has been no studies reported on the influence of the chronic administration of Aconitine. Thus, this study was conducted to investigate the influence of chronic administration of Aconitine in experimental animal models. A dose of 1mg/kg per day was administered to the experimental animal models. We determined the concentration of Aconitine and its metabolites (benzoylaconine and aconine) in organs and blood with gas chromatography/selected ion monitoring (GC/SIM). In addition, we concurrently recorded the electrocardiogram (ECG). Fifteen minutes after administration on day 0, the early Aconitine administered group (acute group) revealed peak organ and blood concentration levels of Aconitine with a gradual decrease, thereafter. The concentration of Aconitine in organs and blood (from days 0 to 22; 90 min after the last administration of Aconitine) gradually decreased according to repeated administration, whereas benzoylaconine and aconine increased. ECG revealed various types of arrhythmias. However, the frequency of arrhythmias remarkably decreased with time and repeated administration of Aconitine. These results indicate two possibilities. First, the increase in the activity of Aconitine metabolism. Secondly, the decrease of effectiveness to the heart due to long-term (chronic) administration of Aconitine.


