Retinoic acidretinoic acid, treats acne vulgaris and keratosis pilaris CAS# 302-79-4 |
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

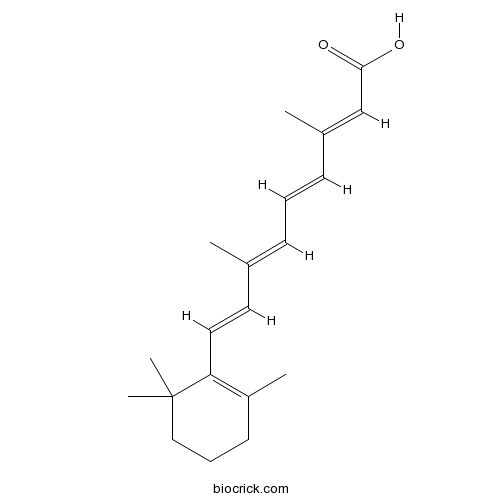
| Cas No. | 302-79-4 | SDF | Download SDF |
| PubChem ID | 444795 | Appearance | Yellow powder |
| Formula | C20H28O2 | M.Wt | 300.44 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Tretinoin, ATRA; Vitamin A Acid | ||
| Solubility | DMSO : ≥ 50 mg/mL (166.42 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenoic acid | ||
| SMILES | CC1=C(C(CCC1)(C)C)C=CC(=CC=CC(=CC(=O)O)C)C | ||
| Standard InChIKey | SHGAZHPCJJPHSC-YCNIQYBTSA-N | ||
| Standard InChI | InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Retinoic acid is a metabolite of vitamin A that plays important roles in cell growth, differentiation, and organogenesis. Retinoic acid is a natural agonist of RAR nuclear receptors, with IC50s of 14 nM for RARα/β/γ. Retinoic acid bind to PPARβ/δ with Kd of 17 nM. It also helps repair Smad3/TGF-β1-induced lung damage in hyperoxic mice. |
| Targets | TGF-β/Smad | ROS | RARα/β/γ |
| In vitro | Identification of novel retinoic acid target genes.[Pubmed: 25251699]Dev Biol. 2014 Nov 15;395(2):199-208.Retinoic acid is required for diverse ontogenic processes and as such identification of the genes and pathways affected by Retinoic acid is critical to understanding these pleiotropic effects. The presomitic mesoderm of the E8.5 mouse embryo is composed of undifferentiated cells that are depleted of Retinoic acid, yet are competent to respond to the retinoid signal. A RARE of hepatic Gck promoter interacts with RARα, HNF4α and COUP-TFII that affect retinoic acid- and insulin-induced Gck expression.[Pubmed: 24973045]J Nutr Biochem. 2014 Sep;25(9):964-76.The expression of hepatic glucokinase gene (Gck) is regulated by hormonal and nutritional signals. How these signals integrate to regulate the hepatic Gck expression is unclear. We have shown that the hepatic Gck expression is affected by Vitamin A status and synergistically induced by insulin and retinoids in primary rat hepatocytes. |
| In vivo | Retinoic acid induced repair in the lung of adult hyperoxic mice, reducing transforming growth factor-β1 (TGF-β1) mediated abnormal alterations.[Pubmed: 24576683]Acta Histochem. 2014 Jun;116(5):810-9.The aim of the study was to determine the effects of Retinoic acid on lung alveolar repair in adult hyperoxic mice and to investigate the relationship between TGF-β1 and Retinoic acid during the repair processes. |
| Kinase Assay | Retinoic acid acts as a selective human IgA switch factor.[Pubmed: 24994461]MicroRNA-302b-inhibited E2F3 transcription factor is related to all trans retinoic acid-induced glioma cell apoptosis.[Pubmed: 25040912]J Neurochem. 2014 Dec;131(6):731-42.All-trans Retinoic acid (ATRA), a derivative of retinoid, is involved in the onset of differentiation and apoptosis in a wide variety of normal and cancer cells. MicroRNAs (miRNAs) are small non-coding RNAs that control gene expression. Several miRNAs were identified to participate in ATRA-mediated cell differentiation. However, no studies have demonstrated whether miRNA can enhance ATRA cytotoxicity, thereby resulting in cell apoptosis. This study investigated the effects of ATRA-mediated miRNA expression in activating apoptotic pathways in glioblastoma. Hum Immunol. 2014 Aug;75(8):923-9.Retinoic acid (RA) is known to have several functions that lead to a potent mucosal IgA response. Nevertheless, its exact role in human IgA synthesis has yet to be elucidated. Thus, we investigated the role of RA in promoting IgA isotype switching in human B cells. |

Retinoic acid Dilution Calculator

Retinoic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3285 mL | 16.6423 mL | 33.2845 mL | 66.569 mL | 83.2113 mL |
| 5 mM | 0.6657 mL | 3.3285 mL | 6.6569 mL | 13.3138 mL | 16.6423 mL |
| 10 mM | 0.3328 mL | 1.6642 mL | 3.3285 mL | 6.6569 mL | 8.3211 mL |
| 50 mM | 0.0666 mL | 0.3328 mL | 0.6657 mL | 1.3314 mL | 1.6642 mL |
| 100 mM | 0.0333 mL | 0.1664 mL | 0.3328 mL | 0.6657 mL | 0.8321 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tretinoin(Aberela) is retinoic acid in pharmaceutical form and used to treat acne vulgaris and keratosis pilaris.
In cultured human mesangial cells, tretinoin increased the reduced glutathione content and the catalase activity in a dose- and time-dependent way, which then prevented the cytotoxicity of H2O2 [1].
In old male Fischer 344 rats, tretinoin inhibited the expression of TNF-β1 and osteopontin (a protein with chemotactic and cell adhesive properties) in the renal cortex [1]. In male NMRI-mice treated with 1×109 sheep red blood cells (SRBCs), tretinoin markedly inhibited cellular immunity and increased humoral immunity. Tretinoin reduced lymphocyte proliferation, NBT reduction and the secretion of interleukin-17, but increased the production of interleukin-10 [2]. In patients with facial acne, clindamycin phosphate Tretinoin Gel (CTG) (1.2% clindamycin phosphate, 0.025% tretinoin in a gel base (Velac)) was significantly more effective than 0.025% tretinoin, which relayed on the anti-inflammatory efficacy of tretinoin [3].
References:
[1]. Manzano VM, Puyol MR, Puyol DR, et al. Tretinoin prevents age-related renal changes and stimulates antioxidant defenses in cultured renal mesangial cells. J Pharmacol Exp Ther, 1999, 289(1): 123-132.
[2]. Froushani SM, Galeh HE. New insight into the immunomodulatory mechanisms of Tretinoin in NMRI mice. Iran J Basic Med Sci, 2014, 17(9): 632-637.
[3]. Richter JR, Förström LR, Kiistala UO, et al. Efficacy of the fixed 1.2% clindamycin phosphate, 0.025% tretinoin gel formulation (Velac) and a proprietary 0.025% tretinoin gel formulation (Aberela) in the topical control of facial acne. J Eur Acad Dermatol Venereol, 1998, 11(3): 227-233.
- DL-Alanine
Catalog No.:BCN8539
CAS No.:302-72-7
- Aconitine
Catalog No.:BCN1014
CAS No.:302-27-2
- Hydroxyprogesterone acetate
Catalog No.:BCC8997
CAS No.:302-23-8
- Desoxyrhaponticin
Catalog No.:BCN2954
CAS No.:30197-14-9
- Ro 5-3335
Catalog No.:BCC7962
CAS No.:30195-30-3
- Boc-Lys(Boc)-OSu
Catalog No.:BCC3415
CAS No.:30189-36-7
- D4476
Catalog No.:BCC1508
CAS No.:301836-43-1
- SB 431542
Catalog No.:BCC3658
CAS No.:301836-41-9
- Jasminin
Catalog No.:BCN7468
CAS No.:30164-93-3
- Compstatin control peptide
Catalog No.:BCC6067
CAS No.:301544-78-5
- Seneganolide
Catalog No.:BCN5209
CAS No.:301530-12-1
- H-Val-NH2.HCl
Catalog No.:BCC3144
CAS No.:3014-80-0
- Arenobufagin 3-hemisuberate
Catalog No.:BCN7837
CAS No.:30219-16-0
- Effusanin A
Catalog No.:BCN5210
CAS No.:30220-43-0
- Ingenol
Catalog No.:BCN2333
CAS No.:30220-46-3
- 1-Hydroxybaccatin I
Catalog No.:BCN5211
CAS No.:30244-37-2
- Potassium 7-hydroxynaphthalene-1-sulfonate
Catalog No.:BCN8289
CAS No.:30252-40-5
- Rosiglitazone HCl
Catalog No.:BCC2269
CAS No.:302543-62-0
- Bisline
Catalog No.:BCN2062
CAS No.:30258-28-7
- 3,4,5-Trimethoxycinnamyl alcohol
Catalog No.:BCN5212
CAS No.:30273-62-2
- TCS 46b
Catalog No.:BCC7482
CAS No.:302799-86-6
- Ciliobrevin A
Catalog No.:BCC3939
CAS No.:302803-72-1
- Ro 01-6128
Catalog No.:BCC7922
CAS No.:302841-86-7
- Ro 67-4853
Catalog No.:BCC7921
CAS No.:302841-89-0
MicroRNA-302b-inhibited E2F3 transcription factor is related to all trans retinoic acid-induced glioma cell apoptosis.[Pubmed:25040912]
J Neurochem. 2014 Dec;131(6):731-42.
All-trans Retinoic acid (ATRA), a derivative of retinoid, is involved in the onset of differentiation and apoptosis in a wide variety of normal and cancer cells. MicroRNAs (miRNAs) are small non-coding RNAs that control gene expression. Several miRNAs were identified to participate in ATRA-mediated cell differentiation. However, no studies have demonstrated whether miRNA can enhance ATRA cytotoxicity, thereby resulting in cell apoptosis. This study investigated the effects of ATRA-mediated miRNA expression in activating apoptotic pathways in glioblastoma. First, we found that high-dose ATRA treatment significantly reduced cell viability, caspase-dependent apoptosis, endoplasmic reticular (ER) stress activation, and intracellular reactive oxygen species accumulation. From microarray data, miR-302b was analyzed as a putative downstream regulator upon ATRA treatment. Furthermore, we found that ATRA up-regulated miR-302b expression in a dose- and time-dependent manner through Retinoic acid receptor alpha-mediated pathway. Overexpression and knockdown of miR-302b significantly influenced ATRA-mediated cytotoxicity. E2F3, an important transcriptional regulator of glioma proliferation, was validated to be a direct target gene of miR-302b. The miR-302b-reduced E2F3 levels were also identified to be associated with ATRA-mediated glioma cell death. These results emphasize that an ATRA-mediated miR-302b network may provide novel therapeutic strategies for glioblastoma therapy. We propose that high-dose all-trans Retinoic acid (ATRA) treatment, a derivative of retinoid, significantly induces glioblastoma cell apoptosis via caspase-dependent apoptosis, endoplasmic reticular (ER) stress, and intracellular reactive oxygen species (ROS) accumulation. The miR-302b overexpression enhanced by ATRA-mediated Retinoic acid receptor (RAR)alpha pathway was also identified. The E2F3 repression, a novel target gene of miR-302b, was involved in ATRA-induced glioblastoma cell cytotoxicity.
Identification of novel retinoic acid target genes.[Pubmed:25251699]
Dev Biol. 2014 Nov 15;395(2):199-208.
Retinoic acid is required for diverse ontogenic processes and as such identification of the genes and pathways affected by Retinoic acid is critical to understanding these pleiotropic effects. The presomitic mesoderm of the E8.5 mouse embryo is composed of undifferentiated cells that are depleted of Retinoic acid, yet are competent to respond to the retinoid signal. We have exploited these properties to use this tissue to identify novel Retinoic acid-responsive genes, including candidate target genes, by treating E8.5 embryos with Retinoic acid and assessing changes in gene expression in the presomitic mesoderm by microarray analysis. This exercise yielded a cohort of genes that were differentially expressed in response to exogenous Retinoic acid exposure. Among these were a number of previously characterized Retinoic acid targets, validating this approach. In addition, we recovered a number of novel candidate target genes which were confirmed as Retinoic acid-responsive by independent analysis. Chromatin immunoprecipitation assays revealed Retinoic acid receptor occupancy of the promoters of certain of these genes. We further confirmed direct Retinoic acid regulation of the F11r gene, a new RA target, using tissue culture models. Our results reveal a significant number of potential RA targets implicated in embryonic development and offer a novel in vivo system for better understanding of retinoid-dependent transcription.
A RARE of hepatic Gck promoter interacts with RARalpha, HNF4alpha and COUP-TFII that affect retinoic acid- and insulin-induced Gck expression.[Pubmed:24973045]
J Nutr Biochem. 2014 Sep;25(9):964-76.
The expression of hepatic glucokinase gene (Gck) is regulated by hormonal and nutritional signals. How these signals integrate to regulate the hepatic Gck expression is unclear. We have shown that the hepatic Gck expression is affected by Vitamin A status and synergistically induced by insulin and retinoids in primary rat hepatocytes. We hypothesized that this is mediated by a Retinoic acid responsive element (RARE) in the hepatic Gck promoter. Here, we identified the RARE in the hepatic Gck promoter using standard molecular biology techniques. The single nucleotide mutations affecting the promoter activation by Retinoic acid (RA) were also determined for detail analysis of protein and DNA interactions. We have optimized experimental conditions for performing electrophoresis mobility shift assay and demonstrated the interactions of the Retinoic acid receptor alpha (RARalpha), retinoid X receptor alpha (RXRalpha), hepatocyte nuclear factor 4alpha (HNF4alpha) and chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) in the rat nuclear extract with this RARE, suggesting their roles in the regulation of Gck expression. Chromatin immunoprecipitation assays demonstrated that recombinant adenovirus-mediated overexpression of RARalpha, HNF4alpha and COUP-TFII, but not RXRalpha, significantly increased their occupancy in the hepatic Gck promoter in primary rat hepatocytes. Overexpression of RARalpha, HNF4alpha and COUP-TFII, but not RXRalpha, also affected the RA- and insulin-mediated Gck expression in primary rat hepatocytes. In summary, this hepatic Gck promoter RARE interacts with RARalpha, HNF4alpha and COUP-TFII to integrate Vitamin A and insulin signals.
Retinoic acid acts as a selective human IgA switch factor.[Pubmed:24994461]
Hum Immunol. 2014 Aug;75(8):923-9.
Retinoic acid (RA) is known to have several functions that lead to a potent mucosal IgA response. Nevertheless, its exact role in human IgA synthesis has yet to be elucidated. Thus, we investigated the role of RA in promoting IgA isotype switching in human B cells. We found that RA increased IgA production and the expression of germ-line IgA1 and IgA2 transcripts (GLTalpha1 and GLTalpha2). This induction occurred alongside an increase in the frequency of IgA1-secreting B cell clones, as assessed by limiting dilution analysis. Under the same conditions, RA did not increase IgM and IgG production. Am80, an agonist of RA receptor alpha (RARalpha), increased IgA production. In addition, RA activity was abrogated by LE540, an antagonist of RAR, suggesting that the RAR pathway is involved in RA-induced IgA production. Taken together, these results indicate that RA induces IgA isotype switching mainly through RARalpha in human B cells.
Retinoic acid induced repair in the lung of adult hyperoxic mice, reducing transforming growth factor-beta1 (TGF-beta1) mediated abnormal alterations.[Pubmed:24576683]
Acta Histochem. 2014 Jun;116(5):810-9.
The aim of the study was to determine the effects of Retinoic acid on lung alveolar repair in adult hyperoxic mice and to investigate the relationship between TGF-beta1 and Retinoic acid during the repair processes. Adult mice were divided into 4 groups. Two groups were given daily intraperitoneal injections of peanut oil/dimethylsulfoxide mixture and Retinoic acid (50mg/kg body weight, 50 mul of volume) dissolved in peanut oil/dimethylsulfoxide mixture for 12 days with a 2-day break on days 6 and 7. Following hyperoxia (100% oxygen) for 72 h the remaining two groups were treated in the same manner as already described: peanut oil/dimethylsulfoxide mixture and Retinoic acid. Lung structure was investigated by light microscopy. TGF-beta1 and Smad protein expressions in the lung were assayed by biochemical methods. Hyperoxic mice exhibited damage to the alveolar walls, increased cell proliferation and induced Smad3/TGF-beta1 signaling. Smad2 and phospho-Smad2 protein expressions were unchanged in all groups. Retinoic acid administration improved the degenerative alterations caused by hyperoxia and helped in alveolar repair. This positive effect of Retinoic acid resulted from the inhibition of Smad3/TGF-beta1 signaling via reduced Smad4 mRNA and increased Smad7 protein expression. Retinoic acid also induced alveolarization and restricted Smad3/TGF-beta1 signaling by decreasing Smad4 mRNA in healthy mice. Thus, Retinoic acid helped repair Smad3/TGF-beta1-induced lung damage in hyperoxic mice.
All-trans retinoic acid is a ligand for the orphan nuclear receptor ROR beta.[Pubmed:12958591]
Nat Struct Biol. 2003 Oct;10(10):820-5.
Retinoids regulate gene expression through binding to the nuclear Retinoic acid receptors (RARs) and retinoid X receptors (RXRs). In contrast, no ligands for the Retinoic acid receptor-related orphan receptors beta and gamma (ROR beta and gamma) have been identified, yet structural data and structure-function analyses indicate that ROR beta is a ligand-regulated nuclear receptor. Using nondenaturing mass spectrometry and scintillation proximity assays we found that all-trans Retinoic acid (ATRA) and several retinoids bind to the ROR beta ligand-binding domain (LBD). The crystal structures of the complex with ATRA and with the synthetic analog ALRT 1550 reveal the binding modes of these ligands. ATRA and related retinoids inhibit ROR beta but not ROR alpha transcriptional activity suggesting that high-affinity, subtype-specific ligands could be designed for the identification of ROR beta target genes. Our results identify ROR beta as a retinoid-regulated nuclear receptor, providing a novel pathway for retinoid action.
Differentiation of embryonic stem cells into adipocytes in vitro.[Pubmed:9202388]
J Cell Sci. 1997 Jun;110 ( Pt 11):1279-85.
Embryonic stem cells, derived from the inner cell mass of murine blastocysts, can be maintained in a totipotent state in vitro. In appropriate conditions embryonic stem cells have been shown to differentiate in vitro into various derivatives of all three primary germ layers. We describe in this paper conditions to induce differentiation of embryonic stem cells reliably and at high efficiency into adipocytes. A prerequisite is to treat early developing embryonic stem cell-derived embryoid bodies with Retinoic acid for a precise period of time. Retinoic acid could not be substituted by adipogenic hormones nor by potent activators of peroxisome proliferator-activated receptors. Treatment with Retinoic acid resulted in the subsequent appearance of large clusters of mature adipocytes in embryoid body outgrowths. Lipogenic and lipolytic activities as well as high level expression of adipocyte specific genes could be detected in these cultures. Analysis of expression of potential adipogenic genes, such as peroxisome proliferator-activated receptors gamma and delta and CCAAT/enhancer binding protein beta, during differentiation of Retinoic acid-treated embryoid bodies has been performed. The temporal pattern of expression of genes encoding these nuclear factors resembled that found during mouse embryogenesis. The differentiation of embryonic stem cells into adipocytes will provide an invaluable model for the characterisation of the role of genes expressed during the adipocyte development programme and for the identification of new adipogenic regulatory genes.
Characterization of human cellular retinoic acid-binding proteins-I and -II: ligand binding affinities and distribution in skin.[Pubmed:8185324]
Arch Biochem Biophys. 1994 May 15;311(1):86-94.
Cellular Retinoic acid-binding proteins (CRABPs) are a family of proteins that specifically bind Retinoic acid (RA) and have been implicated in mediating its action, although their exact function is still unknown. Two CRABPs have been identified and cloned. CRABP-I is present in many tissues and cultured cells; and CRABP-II, first detected in embryonic and neonatal skin of rats and chicks, is now recognized as the predominant form in human epidermis. Previous studies of CRABP protein expression and function could not distinguish between the two forms and perhaps for that reason have yielded conflicting results, particularly with regard to RA-binding affinity in human tissues. In the present study, we have used the FLAG technology to generate recombinant CRABP-II and developed an anion-exchange HPLC assay in order to allow an accurate discrimination of the two proteins. CRABP-II eluted first with a retention time of 6 min, and CRABP-I with a retention time of 14 min. Both CRABP-II and CRABP-I were found to be expressed in human skin, CRABP-II by fibroblasts and keratinocytes and CRABP-I by as yet unidentified cells. This divergent origin supports the hypothesis that CRABP-II and CRABP-I differentially mediate RA effects. Binding studies demonstrated that CRABP-I and CRABP-II possess two classes of RA-binding sites: one class of high-affinity binding sites with a constant of dissociation (Kd) of 1.5 nM for CRABP-I and 4.7 nM for CRABP-II and one class of low-affinity binding sites with a Kd of 69 nM for CRABP-I and 101 nM for CRABP-II. These data further elucidate the complex regulation of retinoid effects in human skin.
A retinoic acid receptor alpha antagonist selectively counteracts retinoic acid effects.[Pubmed:1323127]
Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7129-33.
Retinoic acid (RA) exerts its pleiotropic effects on cell growth and differentiation through the activation of a family of transcription factors-the RA receptors (RARs). Three subtypes of these receptors exist, RAR alpha, RAR beta, and RAR gamma. The receptors are differentially expressed in different cell types and stages of development, suggesting that they may regulate different sets of genes. We have identified a synthetic retinoid with the characteristics of a selective RAR alpha antagonist. This antagonist counteracts RA effects on HL-60 cell differentiation and on B-lymphocyte polyclonal activation. Beyond its potential practical relevance, this and other specific antagonists will be useful to dissect the RAR system and to assign to one given receptor each of the many RA-regulated functions.


