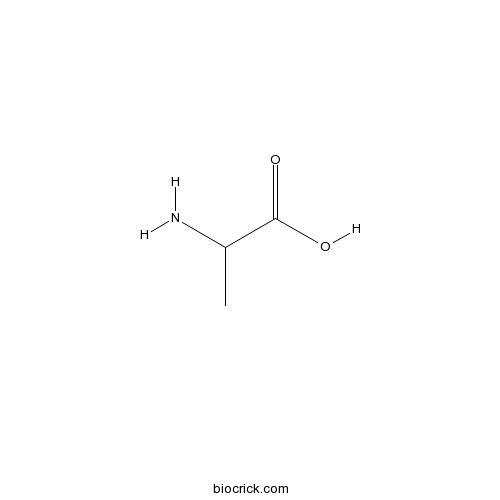
DL-AlanineCAS# 302-72-7 |
- H-D-Abu-OH
Catalog No.:BCC3202
CAS No.:338-69-2
- H-Ala-OH
Catalog No.:BCC3190
CAS No.:56-41-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 302-72-7 | SDF | Download SDF |
| PubChem ID | 602 | Appearance | White needle crystal |
| Formula | C3H7NO2 | M.Wt | 89.09 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-aminopropanoic acid | ||
| SMILES | CC(C(=O)O)N | ||
| Standard InChIKey | QNAYBMKLOCPYGJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C3H7NO2/c1-2(4)3(5)6/h2H,4H2,1H3,(H,5,6) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

DL-Alanine Dilution Calculator

DL-Alanine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 11.2246 mL | 56.123 mL | 112.246 mL | 224.4921 mL | 280.6151 mL |
| 5 mM | 2.2449 mL | 11.2246 mL | 22.4492 mL | 44.8984 mL | 56.123 mL |
| 10 mM | 1.1225 mL | 5.6123 mL | 11.2246 mL | 22.4492 mL | 28.0615 mL |
| 50 mM | 0.2245 mL | 1.1225 mL | 2.2449 mL | 4.4898 mL | 5.6123 mL |
| 100 mM | 0.1122 mL | 0.5612 mL | 1.1225 mL | 2.2449 mL | 2.8062 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Aconitine
Catalog No.:BCN1014
CAS No.:302-27-2
- Hydroxyprogesterone acetate
Catalog No.:BCC8997
CAS No.:302-23-8
- Desoxyrhaponticin
Catalog No.:BCN2954
CAS No.:30197-14-9
- Ro 5-3335
Catalog No.:BCC7962
CAS No.:30195-30-3
- Boc-Lys(Boc)-OSu
Catalog No.:BCC3415
CAS No.:30189-36-7
- D4476
Catalog No.:BCC1508
CAS No.:301836-43-1
- SB 431542
Catalog No.:BCC3658
CAS No.:301836-41-9
- Jasminin
Catalog No.:BCN7468
CAS No.:30164-93-3
- Compstatin control peptide
Catalog No.:BCC6067
CAS No.:301544-78-5
- Seneganolide
Catalog No.:BCN5209
CAS No.:301530-12-1
- H-Val-NH2.HCl
Catalog No.:BCC3144
CAS No.:3014-80-0
- P7C3
Catalog No.:BCC6524
CAS No.:301353-96-8
- Retinoic acid
Catalog No.:BCN2185
CAS No.:302-79-4
- Arenobufagin 3-hemisuberate
Catalog No.:BCN7837
CAS No.:30219-16-0
- Effusanin A
Catalog No.:BCN5210
CAS No.:30220-43-0
- Ingenol
Catalog No.:BCN2333
CAS No.:30220-46-3
- 1-Hydroxybaccatin I
Catalog No.:BCN5211
CAS No.:30244-37-2
- Potassium 7-hydroxynaphthalene-1-sulfonate
Catalog No.:BCN8289
CAS No.:30252-40-5
- Rosiglitazone HCl
Catalog No.:BCC2269
CAS No.:302543-62-0
- Bisline
Catalog No.:BCN2062
CAS No.:30258-28-7
- 3,4,5-Trimethoxycinnamyl alcohol
Catalog No.:BCN5212
CAS No.:30273-62-2
- TCS 46b
Catalog No.:BCC7482
CAS No.:302799-86-6
- Ciliobrevin A
Catalog No.:BCC3939
CAS No.:302803-72-1
- Ro 01-6128
Catalog No.:BCC7922
CAS No.:302841-86-7
Enantiomeric resolution and modeling of DL-alanine-DL-tryptophan dipeptide on amylose stationary phase.[Pubmed:29315810]
Chirality. 2018 Apr;30(4):491-497.
The enantiomeric resolution of DL-Alanine-DL-tryptophan dipeptide is described on amylose stationary phase. The eluent used was CH3 OH horizontal line CH3 COONH4 (10mM) horizontal line CH3 CN (50: 40, 10) at 0.8-mL/min flow, 230-nm detection, 25-minute run time, and 25 degrees C +/- 1 degrees C temperature. The chiral phase was amylose [AmyCoat RP (15 cm x 0.46 cm x 5 micron)]. The magnitudes of the retention factors (k) were 2.71, 3.52, 5.11, and 7.75. The magnitudes of separation factor (alpha) were 1.19, 1.57, and 1.51 while the resolution factors (Rs) were 3.25, 14.84, and 15.76. The limits of detection and quantitation were of 2.5 to 5.4 and 12.8 to 27.5 mug/mL. The enantiomeric resolution is controlled by hydrogen, hydrophobic, pi-pi, steric, etc interactions. The elution order of the enantiomer was supported by the modeling data. The described method is fast, reproducible, precise, and selective, which can be used successfully for evaluating the enantiomers of the reported dipeptide.
Kinetic resolution of N-acetyl-DL-alanine methyl ester using immobilized Escherichia coli cells bearing recombinant esterase from Bacillus cereus.[Pubmed:29676476]
Chirality. 2018 Jul;30(7):907-912.
D-alanine is widely used in medicine, food, additives, cosmetics, and other consumer items. Esterase derived from Bacillus cereus WZZ001 exhibits high hydrolytic activity and stereoselectivity. In this study, we expressed the esterase gene in Escherichia coli BL21 (DE3). We analyzed the biocatalytic resolution of N-acetyl-DL-Alanine methyl ester by immobilized whole E. coli BL21 (DE3) cells, which were prepared through embedding and cross-linking. We analyzed biocatalytic resolution under the optimal conditions of pH of 7.0, temperature of 40 degrees C and substrate concentration of at 700 mM with an enantiomeric excess of 99.99% and e.e.p of 99.50%. The immobilized recombinant B. cereus esterase E. coli BL21 (DE3) cells exhibited excellent reusability and retained 86.04% of their initial activity after 15 cycles of repeated reactions. The immobilized cells are efficient and stable biocatalysts for the preparation of N-acetyl-D-alanine methyl esters.
Cellular Uptake Mechanism of Cationic Branched Polypeptides with Poly[l-Lys] Backbone.[Pubmed:28276242]
ACS Comb Sci. 2017 Apr 10;19(4):246-254.
Cationic macromolecular carriers can be effective carriers for small molecular compounds, drugs, epitopes, or nucleic acids. Polylysine-based polymeric branched polypeptides have been systematically studied on the level of cells and organisms as well. In the present study, we report our findings on the cellular uptake characteristics of nine structurally related polylysine-based polypeptides with cationic side chains composed of (i) single amino acid (poly[Lys(Xi)], XiK) or (ii) oligo[DL-Alanine] (poly[Lys(dl-Alam)], AK) or (iii) oligo[DL-Alanine] with an additional amino acid (X) at the terminal position (poly[Lys(Xi-dl-Alam)] (XAK)) or (iv) at the position next to the polylysine backbone (poly[Lys(dl-Alam-Xi)] (AXK)). In vitro cytotoxicity and cellular uptake were characterized on HT-29 human colon carcinoma and HepG2 human hepatocarcinoma cell lines. Data indicate that the polycationic polypeptides studied are essentially nontoxic in the concentration range studied, and their uptake is very much dependent on the side chain structure (length, identity of amino acid X, and distance between the terminal positive charges) and also on the cell lines. Our findings in uptake inhibition studies suggest that predominantly macropinocytosis and caveole/lipid raft mediated endocytosis are involved. The efficacy of their internalization is markedly influenced by the hydrophobicity and charge properties of the amino acid X. Interestingly, the uptake properties of the these polypeptides show certain similarities to the entry pathways of several cell penetrating peptides.
Production of D-alanine from DL-alanine using immobilized cells of Bacillus subtilis HLZ-68.[Pubmed:28905232]
World J Microbiol Biotechnol. 2017 Sep 13;33(9):176.
Immobilized cells of Bacillus subtilis HLZ-68 were used to produce D-alanine from DL-Alanine by asymmetric degradation. Different compounds such as polyvinyl alcohol and calcium alginate were employed for immobilizing the B. subtilis HLZ-68 cells, and the results showed that cells immobilized using a mixture of these two compounds presented higher L-alanine degradation activity, when compared with free cells. Subsequently, the effects of different concentrations of polyvinyl alcohol and calcium alginate on L-alanine consumption were examined. Maximum L-alanine degradation was exhibited by cells immobilized with 8% (w/v) polyvinyl alcohol and 2% (w/v) calcium alginate. Addition of 400 g of DL-Alanine (200 g at the beginning of the reaction and 200 g after 30 h of incubation) into the reaction solution at 30 degrees C, pH 6.0, aeration of 1.0 vvm, and agitation of 400 rpm resulted in complete L-alanine degradation within 60 h, leaving 185 g of D-alanine in the reaction solution. The immobilized cells were applied for more than 15 cycles of degradation and a maximum utilization rate was achieved at the third cycle. D-alanine was easily extracted from the reaction solution using cation-exchange resin, and the chemical and optical purity of the extracted D-alanine was 99.1 and 99.6%, respectively.
Polar Imperfections in Amino Acid Crystals: Design, Structure, and Emerging Functionalities.[Pubmed:29676901]
Acc Chem Res. 2018 May 15;51(5):1238-1248.
Crystals are physical arrays delineated by polar surfaces and often contain imperfections of a polar nature. Understanding the structure of such defects on the molecular level is of topical importance since they strongly affect the macroscopic properties of materials. Moreover, polar imperfections in crystals can be created intentionally and specifically designed by doping nonpolar crystals with "tailor-made" additives as dopants, since their incorporation generally takes place in a polar mode. Insertion of dopants also induces a polar deformation of neighboring host molecules, resulting in the creation of polar domains within the crystals. The contribution of the distorted host molecules to the polarity of such domains should be substantial, particularly in crystals composed of molecules with large dipole moments, such as the zwitterionic amino acids, which possess dipole moments as high as approximately 14 D. Polar materials are pyroelectric, i.e., they generate surface charge as a result of temperature change. With the application of recent very sensitive instruments for measuring electric currents, coupled with theoretical computations, it has become possible to determine the structure of polar imperfections, including surfaces, at a molecular level. The detection of pyroelectricity requires attachment of electrodes, which might induce various artifacts and modify the surface of the crystal. Therefore, a new method for contactless pyroelectric measurement using X-ray photoelectron spectroscopy was developed and compared to the traditional periodic temperature change technique. Here we describe the molecular-level determination of the structure of imperfections of different natures in molecular crystals and how they affect the macroscopic properties of the crystals, with the following specific examples: (i) Experimental support for the nonclassical crystal growth mechanism as provided by the detection of pyroelectricity from near-surface solvated polar layers present at different faces of nonpolar amino acid crystals. (ii) Enantiomeric disorder in DL-Alanine crystals disclosed by detection of anomalously strong pyroelectricity along their nonpolar directions. The presence of such disorder, which is not revealed by accurate diffraction techniques, explains the riddle of their needlelike morphology. (iii) The design of mixed polar crystals of l-asparagine.H2O/l-aspartic acid with controlled degrees of polarity, as determined by pyroelectricity and X-ray diffraction, and their use in mechanistic studies of electrofreezing of supercooled water. (iv) Pyroelectricity coupled with dispersion-corrected density functional theory calculations and molecular dynamics simulations as an analytical method for the molecular-level determination of the structure of polar domains created by doping of alpha-glycine crystals with different l-amino acids at concentrations below 0.5%. (v) Selective insertion of minute amounts of alcohols within the bulk of alpha-glycine crystals, elucidating their role as inducers of the metastable beta-glycine polymorph. In conclusion, the various examples demonstrate that although these imperfections are present in minute amounts, they can be detected by the sensitive pyroelectric measurement, and by combining them with theoretical computations one can elucidate their diverse emerging functionalities.


