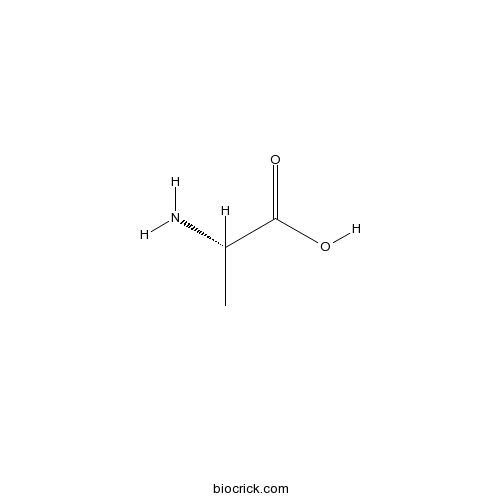
H-Ala-OHGlycine receptor agonist CAS# 56-41-7 |
- Hydroxyfasudil
Catalog No.:BCC1635
CAS No.:105628-72-6
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- Hydroxyfasudil hydrochloride
Catalog No.:BCC1636
CAS No.:155558-32-0
- H-1152 dihydrochloride
Catalog No.:BCC1616
CAS No.:871543-07-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 56-41-7 | SDF | Download SDF |
| PubChem ID | 5950 | Appearance | White powder |
| Formula | C3H7NO2 | M.Wt | 89.1 |
| Type of Compound | Nitrogen-containing Compounds | Storage | Desiccate at -20°C |
| Synonyms | L-alanine; 56-41-7; Alanine; L-alpha-alanine; (S)-Alanine | ||
| Solubility | H2O : 150 mg/mL (1683.69 mM; Need ultrasonic and warming) | ||
| Chemical Name | (2S)-2-aminopropanoic acid | ||
| SMILES | CC(C(=O)O)N | ||
| Standard InChIKey | QNAYBMKLOCPYGJ-REOHCLBHSA-N | ||
| Standard InChI | InChI=1S/C3H7NO2/c1-2(4)3(5)6/h2H,4H2,1H3,(H,5,6)/t2-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Agonist at the inhibitory glycine receptor. |

H-Ala-OH Dilution Calculator

H-Ala-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 11.2233 mL | 56.1167 mL | 112.2334 mL | 224.4669 mL | 280.5836 mL |
| 5 mM | 2.2447 mL | 11.2233 mL | 22.4467 mL | 44.8934 mL | 56.1167 mL |
| 10 mM | 1.1223 mL | 5.6117 mL | 11.2233 mL | 22.4467 mL | 28.0584 mL |
| 50 mM | 0.2245 mL | 1.1223 mL | 2.2447 mL | 4.4893 mL | 5.6117 mL |
| 100 mM | 0.1122 mL | 0.5612 mL | 1.1223 mL | 2.2447 mL | 2.8058 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
H-Ala-OH
- H-Gly-OH
Catalog No.:BCC2946
CAS No.:56-40-6
- Tetraethylammonium chloride
Catalog No.:BCC7554
CAS No.:56-34-8
- Cantharidin
Catalog No.:BCN1280
CAS No.:56-25-7
- Cystamine dihydrochloride
Catalog No.:BCC6344
CAS No.:56-17-7
- 4-Aminobutanoic acid
Catalog No.:BCN2187
CAS No.:56-12-2
- 2,4-Diamino-6-hydroxypyrimidine
Catalog No.:BCC6658
CAS No.:56-06-4
- Methylthiouracil
Catalog No.:BCC4800
CAS No.:56-04-2
- Nitazoxanide
Catalog No.:BCC3824
CAS No.:55981-09-4
- ARC 239 dihydrochloride
Catalog No.:BCC6851
CAS No.:55974-42-0
- Pseudohypericin
Catalog No.:BCN6348
CAS No.:55954-61-5
- Methylpheophorbide A
Catalog No.:BCN7998
CAS No.:5594-30-9
- Betamethasone 17,21-dipropionate
Catalog No.:BCC8875
CAS No.:5593-20-4
- H-Ser-OH
Catalog No.:BCC3028
CAS No.:56-45-1
- Deoxycorticosterone acetate
Catalog No.:BCC4655
CAS No.:56-47-3
- Diethylstilbestrol
Catalog No.:BCC4900
CAS No.:56-53-1
- Quinidine
Catalog No.:BCC7863
CAS No.:56-54-2
- DL-5-Hydroxytryptophan
Catalog No.:BCN1232
CAS No.:56-69-9
- Chloramphenicol
Catalog No.:BCC1201
CAS No.:56-75-7
- Glycerol
Catalog No.:BCC8990
CAS No.:56-81-5
- H-Asp-OH
Catalog No.:BCC2881
CAS No.:56-84-8
- L-Glutamine
Catalog No.:BCC3803
CAS No.:56-85-9
- L-Glutamic acid
Catalog No.:BCN3809
CAS No.:56-86-0
- L-lysine
Catalog No.:BCN7157
CAS No.:56-87-1
- (H-Cys-OH)2
Catalog No.:BCC2915
CAS No.:56-89-3
Hydrolytic cleavage of pyroglutamyl-peptide bond. III. A highly selective cleavage in 70% methanesulfonic acid.[Pubmed:8945768]
Chem Pharm Bull (Tokyo). 1996 Nov;44(11):2033-6.
A method for highly selective cleavage of pGlu-peptide linkages in 70% methanesulfonic acid (MSA) is described. When pGlu-Ala-Phe-OH, pGlu-His-Pro-Oh and dog neuromedin U-8 (d-NMU-8) (1--7)-OH (pGlu-Phe-Leu-Phe-Arg-OH) were hydrolyzed in 70% MSA at 60 degrees C for 3 h or at 25 degrees C for 3 d, the pGlu-peptide linkage was predominantly cleaved to give H-Ala-OH, H-His-Pro-OH and H-Phe-Leu-Phe-Arg-Pro-Arg-OH, in high yields. The results indicated that pGlu-peptide linkages are highly susceptible to 70% MSA, whereas the amide bond of the pyrrolidone moiety of the pGlu residue and other internal peptide bonds are extremely resistant.
Pharmacology of the inhibitory glycine receptor: agonist and antagonist actions of amino acids and piperidine carboxylic acid compounds.[Pubmed:7476923]
Mol Pharmacol. 1995 Nov;48(5):919-27.
To define structure-activity relations for ligands binding to the inhibitory glycine receptor (GlyR), the agonistic and antagonistic properties of alpha- and beta-amino acids were analyzed at the recombinant human alpha 1 GlyR expressed in Xenopus oocytes. The agonistic activity of alpha-amino acids exhibited a marked stereoselectivity and was highly susceptible to substitutions at the C alpha-atom. In contrast, alpha-amino acid antagonism was not enantiomer dependent and was influenced little by C alpha-atom substitutions. The beta-amino acids taurine, beta-aminobutyric acid (beta-ABA), and beta-aminoisobutyric acid (beta-AIBA) are partial agonists at the GlyR. Low concentrations of these compounds competitively inhibited glycine responses, whereas higher concentrations elicited a significant membrane current. Nipecotic acid, which contains a trans-beta-amino acid configuration, behaved as purely competitive GlyR antagonist. Our data are consistent with the existence of a common binding site for all amino acid agonists and antagonists, at which the functional consequences of binding depend on the particular conformation a given ligand adopts within the binding pocket. In the case of beta-amino acids, the trans conformation appears to mediate antagonistic receptor binding, and the cis conformation appears to mediate agonistic receptor binding. This led us to propose that the partial agonist activity of a given beta-amino acid is determined by the relative mole fractions of the respective cis/trans conformers.
A novel antagonist, phenylbenzene omega-phosphono-alpha-amino acid, for strychnine-sensitive glycine receptors in the rat spinal cord.[Pubmed:7812607]
Br J Pharmacol. 1994 Sep;113(1):165-70.
1. 3-[2'-Phosphonomethyl[1,1'-biphenyl]-3-yl]alanine (PMBA) is a novel glycine antagonist at strychnine-sensitive receptors. The chemical structure of PMBA, possessing both a glycine moiety and a phosphono group, is quite different from that of strychnine. 2. In the spinal motoneurone of newborn rats, glycine (100 microM-1 mM) induced depolarizing responses in a concentration-dependent manner. PMBA effectively inhibited depolarizing responses to glycine and other agonists, such as taurine and beta-alanine. The dose-response curves for glycine were shifted to the right in an almost parallel manner (pA2 value: 5.30 +/- 0.23, n = 5) by PMBA which was about 60 times less potent than strychnine (pA2 value: 7.08 +/- 0.21, n = 5) as a glycine antagonist. 3. PMBA (1-100 microM) did not interact with modulatory glycine sites on N-methyl-D-aspartate (NMDA) receptors, which suggests a high selectivity of PMBA for strychnine-sensitive glycine receptors. At considerably high concentrations (0.1 mM-1 mM), PMBA depressed responses to GABA (pA2 value: 3.57 +/- 0.24, n = 3). 4. PMBA inhibited the binding of [3H]-strychnine to synaptosomes from adult rat spinal cords; the IC50 values of PMBA, glycine and strychnine were 8 +/- 2, 9 +/- 3 and 0.08 +/- 0.04 microM, respectively (n = 5) for [3H]-strychnine (4.8 nM). 5. PMBA is a central excitant drug with relatively high potency and selectivity and should be useful as a pharmacological probe for analysing the mechanisms underlying physiological functions of glycine receptors.


