H-Gly-OHEndogenous glycine receptor agonist CAS# 56-40-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 56-40-6 | SDF | Download SDF |
| PubChem ID | 750 | Appearance | Powder |
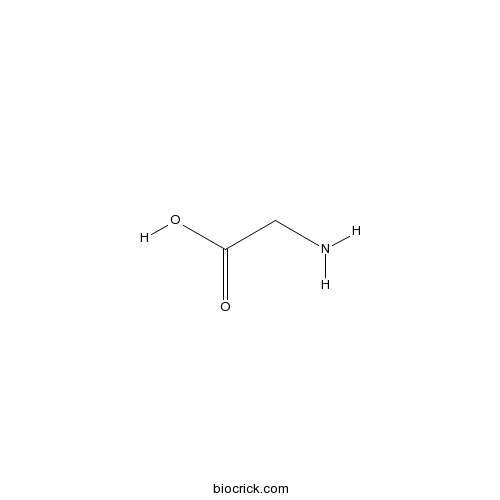
| Formula | C2H5NO2 | M.Wt | 75.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 6 mg/mL (79.93 mM; Need ultrasonic) | ||
| Chemical Name | 2-aminoacetic acid | ||
| SMILES | C(C(=O)O)N | ||
| Standard InChIKey | DHMQDGOQFOQNFH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C2H5NO2/c3-1-2(4)5/h1,3H2,(H,4,5) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | One of the major inhibitory neurotransmitters in the mammalian CNS, predominantly active in the spinal cord and brain stem. Also acts as a modulator of excitatory amino acid transmission mediated by NMDA receptors. Also available as part of the NMDA Receptor - Glycine Site. |

H-Gly-OH Dilution Calculator

H-Gly-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 13.3156 mL | 66.5779 mL | 133.1558 mL | 266.3116 mL | 332.8895 mL |
| 5 mM | 2.6631 mL | 13.3156 mL | 26.6312 mL | 53.2623 mL | 66.5779 mL |
| 10 mM | 1.3316 mL | 6.6578 mL | 13.3156 mL | 26.6312 mL | 33.2889 mL |
| 50 mM | 0.2663 mL | 1.3316 mL | 2.6631 mL | 5.3262 mL | 6.6578 mL |
| 100 mM | 0.1332 mL | 0.6658 mL | 1.3316 mL | 2.6631 mL | 3.3289 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
H-Gly-OH
- Tetraethylammonium chloride
Catalog No.:BCC7554
CAS No.:56-34-8
- Cantharidin
Catalog No.:BCN1280
CAS No.:56-25-7
- Cystamine dihydrochloride
Catalog No.:BCC6344
CAS No.:56-17-7
- 4-Aminobutanoic acid
Catalog No.:BCN2187
CAS No.:56-12-2
- 2,4-Diamino-6-hydroxypyrimidine
Catalog No.:BCC6658
CAS No.:56-06-4
- Methylthiouracil
Catalog No.:BCC4800
CAS No.:56-04-2
- Nitazoxanide
Catalog No.:BCC3824
CAS No.:55981-09-4
- ARC 239 dihydrochloride
Catalog No.:BCC6851
CAS No.:55974-42-0
- Pseudohypericin
Catalog No.:BCN6348
CAS No.:55954-61-5
- Methylpheophorbide A
Catalog No.:BCN7998
CAS No.:5594-30-9
- Betamethasone 17,21-dipropionate
Catalog No.:BCC8875
CAS No.:5593-20-4
- Pennogenin 3-O-beta-chacotrioside
Catalog No.:BCN6707
CAS No.:55916-52-4
- H-Ala-OH
Catalog No.:BCC3190
CAS No.:56-41-7
- H-Ser-OH
Catalog No.:BCC3028
CAS No.:56-45-1
- Deoxycorticosterone acetate
Catalog No.:BCC4655
CAS No.:56-47-3
- Diethylstilbestrol
Catalog No.:BCC4900
CAS No.:56-53-1
- Quinidine
Catalog No.:BCC7863
CAS No.:56-54-2
- DL-5-Hydroxytryptophan
Catalog No.:BCN1232
CAS No.:56-69-9
- Chloramphenicol
Catalog No.:BCC1201
CAS No.:56-75-7
- Glycerol
Catalog No.:BCC8990
CAS No.:56-81-5
- H-Asp-OH
Catalog No.:BCC2881
CAS No.:56-84-8
- L-Glutamine
Catalog No.:BCC3803
CAS No.:56-85-9
- L-Glutamic acid
Catalog No.:BCN3809
CAS No.:56-86-0
- L-lysine
Catalog No.:BCN7157
CAS No.:56-87-1
Why Are B ions stable species in peptide spectra?[Pubmed:24214067]
J Am Soc Mass Spectrom. 1995 Dec;6(12):1165-74.
Protonated amino acids and derivatives RCH(NH2)C(+O)X . H(+) (X = OH, NH2, OCH3) do not form stable acylium ions on loss of HX, but rather the acylium ion eliminates CO to form the immonium ion RCH = NH 2 (+) . By contrast, protonated dipeptide derivatives H2NCH(R)C(+O)NHCH(R')C(+O)X . H(+) [X = OH, OCH3, NH2, NHCH(R'')COOH] form stable B2 ions by elimination of HX. These B2 ions fragment on the metastable ion time scale by elimination of CO with substantial kinetic energy release (T 1/2 = 0.3-0.5 eV). Similarly, protonated N-acetyl amino acid derivatives CH3C(+O)NHCH(R')C(+O)X . H(+) [X = OH, OCH3, NH2, NHCH(R'')COOH] form stable B ions by loss of HX. These B ions also fragment unimolecularly by loss of CO with T 1/2 values of approximately 0.5 eV. These large kinetic energy releases indicate that a stable configuration of the B ions fragments by way of activation to a reacting configuration that is higher in energy than the products, and some of the fragmentation exothermicity of the final step is partitioned into kinetic energy of the separating fragments. We conclude that the stable configuration is a protonated oxazolone, which is formed by interaction of the developing charge (as HX is lost) with the N-terminus carbonyl group and that the reacting configuration is the acyclic acylium ion. This conclusion is supported by the similar fragmentation behavior of protonated 2-phenyl-5-oxazolone and the B ion derived by loss of H-Gly-OH from protonated C6H5C(+O)-Gly-Gly-OH. In addition, ab initio calculations on the simplest B ion, nominally HC(+O)NHCH2CO(+), show that the lowest energy structure is the protonated oxazolone. The acyclic acylium isomer is 1.49 eV higher in energy than the protonated oxazolone and 0.88 eV higher in energy than the fragmentation products, HC(+O)N(+)H = CH2 + CO, which is consistent with the kinetic energy releases measured.
Fragmentation reactions of protonated peptides containing glutamine or glutamic acid.[Pubmed:12577284]
J Mass Spectrom. 2003 Feb;38(2):174-87.
A variety of protonated dipeptides and tripeptides containing glutamic acid or glutamine were prepared by electrospray ionization or by fast atom bombardment ionization and their fragmentation pathways elucidated using metastable ion studies, energy-resolved mass spectrometry and triple-stage mass spectrometry (MS(3)) experiments. Additional mechanistic information was obtained by exchanging the labile hydrogens for deuterium. Protonated H-Gln-Gly-OH fragments by loss of NH(3) and loss of H(2)O in metastable ion fragmentation; under collision-induced dissociation (CID) conditions loss of H-Gly-OH + CO from the [MH - NH(3)](+) ion forms the base peak C(4)H(6)NO(+) (m/z 84). Protonated dipeptides with an alpha-linkage, H-Glu-Xxx-OH, are characterized by elimination of H(2)O and by elimination of H-Xxx-OH plus CO to form the glutamic acid immonium ion of m/z 102. By contrast, protonated dipeptides with a gamma-linkage, H-Glu(Xxx-OH)-OH, do not show elimination of H(2)O or formation of m/z 102 but rather show elimination of NH(3), particularly in metastable ion fragmentation, and elimination of H-Xxx-OH to form m/z 130. Both the alpha- and gamma-dipeptides show formation of [H-Xxx-OH]H(+), with this reaction channel increasing in importance as the proton affinity (PA) of H-Xxx-OH increases. The characteristic loss of H(2)O and formation of m/z 102 are observed for the protonated alpha-tripeptide H-Glu-Gly-Phe-OH whereas the protonated gamma-tripeptide H-Glu(Gly-Gly-OH)-OH shows loss of NH(3) and formation of m/z 130 as observed for dipeptides with the gamma-linkage. Both tripeptides show abundant formation of the y(2)'' ion under CID conditions, presumably because a stable anhydride neutral structure can be formed. Under metastable ion conditions protonated dipeptides of structure H-Xxx-Glu-OH show abundant elimination of H(2)O whereas those of structure H-Xxx-Gln-OH show abundant elimination of NH(3). The importance of these reaction channels is much reduced under CID conditions, the major fragmentation mode being cleavage of the amide bond to form either the a(1) ion or the y(1)'' ion. Particularly when Xxx = Gly, under CID conditions the initial loss of NH(3) from the glutamine containing dipeptide is followed by elimination of a second NH(3) while the initial loss of H(2)O from the glutamic acid dipeptide is followed by elimination of NH(3). Isotopic labelling shows that predominantly labile hydrogens are lost in both steps. Although both [H-Gly-Glu-Gly-OH]H(+) and [H-Gly-Gln-Gly-OH]H(+) fragment mainly to form b(2) and a(2) ions, the latter also shows elimination of NH(3) plus a glycine residue and formation of protonated glycinamide. Isotopic labelling shows extensive mixing of labile and carbon-bonded hydrogens in the formation of protonated glycinamide.
Glycine buffered synthesis of layered iron(II)-iron(III) hydroxides (green rusts).[Pubmed:27894515]
J Colloid Interface Sci. 2017 Jul 1;497:429-438.
Layered Fe(II)-Fe(III) hydroxides (green rusts, GRs) are efficient reducing agents against oxidizing contaminants such as chromate, nitrate, selenite, and nitroaromatic compounds and chlorinated solvents. In this study, we adopted a buffered precipitation approach where glycine (GLY) was used in the synthesis of sulfate-interlayered GR (GRSO4) by aerial oxidation of Fe(II) or co-precipitation by adding Fe(III) salt to an aqueous solution of Fe(II) at constant pH. In both the oxidation and the co-precipitation methods pure crystalline GRSO4 was precipitated in the presence of 70mM GLY (pH 8.0), whereas in the absence of GLY, synthesis failed under similar conditions. Gycine functions as both a pH buffer and a ligand; Fe(II)-GLY complexes serve as a source of base (Fe(II)-GLY+H2O-->Fe(II)+H-GLY+OH(-)) during GR formation, supplying about 45% of the total base required for the synthesis. The GLY buffer decreases pH fluctuations during base addition and hence allows for fast GRSO4 precipitation, minimizing byproduct formation. The use of other pH buffers [4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid and 2-amino-2-(hydroxymethyl)-1,3-propanediol] was also tested but failed. Mossbauer spectroscopy, X-ray diffraction, Fourier transform infrared, transmission electron microscopy, and Fe(II) measurements confirmed the purity, stoichiometry, and pyroaurite-type structure of the obtained GRSO4. The formula of GRSO4 was found to be Fe(II)4.08Fe(III)1.98(OH)11.6(SO4)1.00, and the tabular GR crystals had a lateral size of 100-500nm and a thickness of about 40nm. Upscaling of the synthesis by either 25 times in volume or 20 times in Fe(II) concentration resulted in pure GRSO4 products. Compared with the conventional unbuffered GRSO4 synthesis method, the present method can provide pure products with a controllable, fast, and low-cost process.
Glycine receptors: heterogeneous and widespread in the mammalian brain.[Pubmed:1722365]
Trends Neurosci. 1991 Oct;14(10):458-61.
The amino acid glycine is an established inhibitory neurotransmitter in the spinal cord and brain stem. Its postsynaptic receptor has been purified, and cDNAs of the receptor subunits have been cloned and sequenced. Recent molecular studies indicate considerable diversity of the inhibitory glycine receptor (GlyR); this diversity stems from both the existence of several alpha-subunit genes encoding isoforms of distinct pharmacology, and alternative splicing of their primary transcripts. In situ hybridization studies reveal a widespread expression of GlyRs throughout the mammalian CNS, suggesting that glycinergic neurotransmission may be implicated in many higher brain functions.


