2,4-Diamino-6-hydroxypyrimidineGTP cyclohydrolase I (GCH1) inhibitor CAS# 56-06-4 |
- (24R)-MC 976
Catalog No.:BCC1289
CAS No.:112828-09-8
- (24S)-MC 976
Catalog No.:BCC1291
CAS No.:112849-14-6
- 1alpha, 25-Dihydroxy VD2-D6
Catalog No.:BCC1299
CAS No.:216244-04-1
- 1alpha, 24, 25-Trihydroxy VD2
Catalog No.:BCC1298
CAS No.:457048-34-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 56-06-4 | SDF | Download SDF |
| PubChem ID | 2944 | Appearance | Powder |
| Formula | C4H6N4O | M.Wt | 126.12 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >5.1mg/mL in DMSO | ||
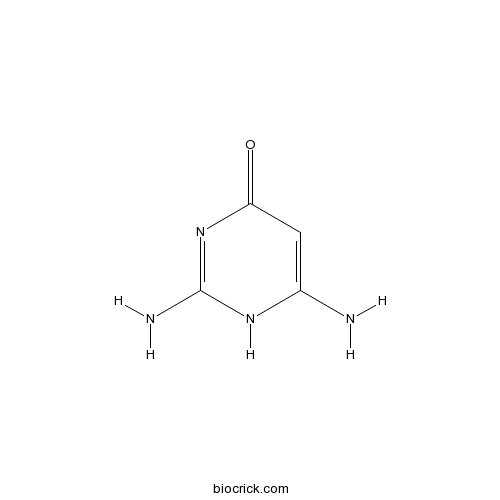
| Chemical Name | 2,6-diamino-1H-pyrimidin-4-one | ||
| SMILES | C1=C(NC(=NC1=O)N)N | ||
| Standard InChIKey | SWELIMKTDYHAOY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C4H6N4O/c5-2-1-3(9)8-4(6)7-2/h1H,(H5,5,6,7,8,9) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GTP cyclohydrolase I (GCH1) inhibitor. Prevents the de novo synthesis of tetrahydrobiopterin (BH4) and thus suppresses the activity of NO synthase. Exhibits more potent inhibition of GCH1 in the presence of GFRP (GTP cyclohydrolase feedback-regulatory protein). |

2,4-Diamino-6-hydroxypyrimidine Dilution Calculator

2,4-Diamino-6-hydroxypyrimidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.929 mL | 39.6448 mL | 79.2896 mL | 158.5791 mL | 198.2239 mL |
| 5 mM | 1.5858 mL | 7.929 mL | 15.8579 mL | 31.7158 mL | 39.6448 mL |
| 10 mM | 0.7929 mL | 3.9645 mL | 7.929 mL | 15.8579 mL | 19.8224 mL |
| 50 mM | 0.1586 mL | 0.7929 mL | 1.5858 mL | 3.1716 mL | 3.9645 mL |
| 100 mM | 0.0793 mL | 0.3964 mL | 0.7929 mL | 1.5858 mL | 1.9822 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Methylthiouracil
Catalog No.:BCC4800
CAS No.:56-04-2
- Nitazoxanide
Catalog No.:BCC3824
CAS No.:55981-09-4
- ARC 239 dihydrochloride
Catalog No.:BCC6851
CAS No.:55974-42-0
- Pseudohypericin
Catalog No.:BCN6348
CAS No.:55954-61-5
- Methylpheophorbide A
Catalog No.:BCN7998
CAS No.:5594-30-9
- Betamethasone 17,21-dipropionate
Catalog No.:BCC8875
CAS No.:5593-20-4
- Pennogenin 3-O-beta-chacotrioside
Catalog No.:BCN6707
CAS No.:55916-52-4
- Polyphyllin VI
Catalog No.:BCN1053
CAS No.:55916-51-3
- Cucurbitacin R
Catalog No.:BCN7877
CAS No.:55903-92-9
- Friedelin
Catalog No.:BCN5747
CAS No.:559-74-0
- beta-Amyrin
Catalog No.:BCN5746
CAS No.:559-70-6
- Morolic acid
Catalog No.:BCN7475
CAS No.:559-68-2
- 4-Aminobutanoic acid
Catalog No.:BCN2187
CAS No.:56-12-2
- Cystamine dihydrochloride
Catalog No.:BCC6344
CAS No.:56-17-7
- Cantharidin
Catalog No.:BCN1280
CAS No.:56-25-7
- Tetraethylammonium chloride
Catalog No.:BCC7554
CAS No.:56-34-8
- H-Gly-OH
Catalog No.:BCC2946
CAS No.:56-40-6
- H-Ala-OH
Catalog No.:BCC3190
CAS No.:56-41-7
- H-Ser-OH
Catalog No.:BCC3028
CAS No.:56-45-1
- Deoxycorticosterone acetate
Catalog No.:BCC4655
CAS No.:56-47-3
- Diethylstilbestrol
Catalog No.:BCC4900
CAS No.:56-53-1
- Quinidine
Catalog No.:BCC7863
CAS No.:56-54-2
- DL-5-Hydroxytryptophan
Catalog No.:BCN1232
CAS No.:56-69-9
- Chloramphenicol
Catalog No.:BCC1201
CAS No.:56-75-7
DFT simulations and vibrational analysis of FT-IR and FT-Raman spectra of 2,4-diamino-6-hydroxypyrimidine.[Pubmed:19406685]
Spectrochim Acta A Mol Biomol Spectrosc. 2009 Aug 15;73(4):642-9.
Quantum mechanical calculations of energies, geometries and vibrational wavenumbers of 2,4-Diamino-6-hydroxypyrimidine (2,4DA6HP) were carried out by using ab initio HF and density functional theory (DFT/B3LYP) method using 6-311G(d,p) basis set. The optimized geometrical parameters obtained by B3LYP method show good agreement with experimental X-ray data. The best level of theory in order to reproduce the experimental wavenumbers is B3LYP method with the 6-311G(d,p) basis set. The difference between the observed and scaled wavenumber values of most of the fundamentals is very small. A detailed interpretation of the infrared spectra of 2,4DA6HP was also reported. The calculated HOMO and LUMO energies show that charge transfer occurs in the molecule. The entropy of the title compound is also performed at HF/6-311G(d,p) and B3LYP/6-311G(d,p) levels of theory. The theoretical spectrograms for FT-IR and FT-Raman spectra of the title molecule have been constructed.
2,4-Diamino-6-Hydroxypyrimidine Based Poly(azomethine-Urethane): Synthesis and Application as a Fluorescent Probe for Detection of Cu(2+) in Aqueous Solution.[Pubmed:26169377]
J Fluoresc. 2015 Sep;25(5):1339-49.
A novel poly(azomethine-urethane)-based 2,4-diamino-6-hydroxyprimidine was synthesized with chemical reaction and it designed as fluorescence probe for determination of Cu(2+) in aqueous solution. The photoluminescence (PL) characteristic of the prepared Schiff base (HPAMP) and its poly(azomethine-urethane) (P-HPAMP) derivative were investigated in different polarity solvents suh as MeOH, THF and DMF. PL measurements showed that both HPAMP and P-HPAMP have higher emission intensity and Stoke's shift value (DeltalambdaST) in THF than the other solvents. Also, the proposed probe exhibited a specific fluorescent on response to Cu(2+) over the other tested transition metal ions in aqueous solution. The sensor gave highly selective and sensetive response against Cu(2+) as increasing a new emission peak at 341 nm, and possible interference and quenching effect of the other tested transition metal ions were found too low. Detection limit of Cu(2+) sensor was also calculated as 7.87 x 10(-6) mol L(-1) in THF/deionized water (1:2, v:v).
2,4-Diamino-6-hydroxypyrimidine (DAHP) suppresses cytokine-induced VCAM-1 expression on the cell surface of human umbilical vein endothelial cells in a BH(4)-independent manner.[Pubmed:18423409]
Biochim Biophys Acta. 2008 Jul-Aug;1780(7-8):960-5.
2,4-Diamino-6-hydroxypyrimidine (DAHP) is considered a specific inhibitor of BH(4) biosynthesis and is widely used in order to elucidate the possible biological function of BH(4) in various cells. In the present study, we found that both the synthesis of tetrahydrobiopterin (BH(4)) and expression of vascular cell adhesion molecule 1 (VCAM-1) were increased in human umbilical vein endothelial cells (HUVEC) treated with proinflammatory cytokines. Thus we examined the effects of DAHP to clarify whether BH(4) might be involved in the expression of VCAM-1 in HUVEC. DAHP reduced the levels of both BH(4) and VCAM-1 induced by TNF-alpha and IFN-gamma. However, the dose-response curves of DAHP for the suppression of the VCAM-1 level and that of BH(4) level were markedly different. Supplementation with sepiapterin failed to restore the depressed VCAM-1 level, although it completely restored the BH(4) level. Furthermore, DAHP significantly reduced the VCAM-1 level under the experimental conditions using TNF-alpha alone, which failed to induce BH(4) production. Taken together, these results indicate that DAHP inhibited the expression of VCAM-1 in a BH(4)-independent manner in HUVEC. In the present study, we also found that DAHP significantly suppressed the accumulation of cytokine-induced NF-kappaB (p65) in the nucleus as well as the mRNA levels of VCAM-1 and GTP cyclohydrolase I (GTPCH), the rate-limiting enzyme of BH(4) synthesis. The data obtained in this study suggest that DAHP reduced VCAM-1 and GTPCH protein synthesis at least partially via suppressing the NF-kappaB level in the nucleus of HUVEC.
The mechanism of potent GTP cyclohydrolase I inhibition by 2,4-diamino-6-hydroxypyrimidine: requirement of the GTP cyclohydrolase I feedback regulatory protein.[Pubmed:15292175]
J Biol Chem. 2004 Sep 24;279(39):40677-82.
Inhibition of GTP cyclohydrolase I (GTPCH) has been used as a selective tool to assess the role of de novo synthesis of (6R)-5,6,7,8-tetrahydro-L-biopterin (BH4) in a biological system. Toward this end, 2,4-Diamino-6-hydroxypyrimidine (DAHP) has been used as the prototypical GTPCH inhibitor. Using a novel real-time kinetic microplate assay for GTPCH activity and purified prokaryote-expressed recombinant proteins, we show that potent inhibition by DAHP is not the result of a direct interaction with GTPCH. Rather, inhibition by DAHP in phosphate buffer occurs via an indirect mechanism that requires the presence of GTPCH feedback regulatory protein (GFRP). Notably, GFRP was previously discovered as the essential factor that reconstitutes inhibition of pure recombinant GTPCH by the pathway end product BH4. Thus, DAHP inhibits GTPCH by engaging the endogenous feedback inhibitory system. We further demonstrate that L-Phe fully reverses the inhibition of GTPCH by DAHP/GFRP, which is also a feature in common with inhibition by BH4/GFRP. These findings suggest that DAHP is not an indiscriminate inhibitor of GTPCH in biological systems; instead, it is predicted to preferentially attenuate GTPCH activity in cells that most abundantly express GFRP and/or contain the lowest levels of L-Phe.
GTP cyclohydrolase I inhibition by the prototypic inhibitor 2, 4-diamino-6-hydroxypyrimidine. Mechanisms and unanticipated role of GTP cyclohydrolase I feedback regulatory protein.[Pubmed:9694862]
J Biol Chem. 1998 Aug 14;273(33):21091-8.
2,4-Diamino-6-hydroxypyrimidine (DAHP) is considered to be a selective and direct-acting inhibitor of GTP cyclohydrolase I (GTPCH), the first and rate-limiting enzyme in the pathway for synthesis of tetrahydrobiopterin (BH4). Accordingly, DAHP has been widely employed to distinguish whether de novo BH4 synthesis is required in a given biological system. Although it has been assumed that DAHP inhibits GTPCH by direct competition with substrate GTP, this has never been formally demonstrated. In view of apparent structural homology between DAHP and BH4, we questioned whether DAHP may mimic BH4 in its inhibition of GTPCH by an indirect mechanism, involving interaction with a recently cloned 9.5-kDa protein termed GTPCH Feedback Regulatory Protein (GFRP). We show by reverse transcription-polymerase chain reaction that GFRP mRNA is constitutively expressed in rat aortic smooth muscle cells and further induced by treatment with immunostimulants. Moreover, functional GFRP is expressed and immunostimulant-induced BH4 accumulates in sufficient quantity to trigger feedback inhibition of GTPCH. Studies with DAHP reveal that GFRP is also essential to achieve potent inhibition of GTPCH. Indeed, DAHP inhibits GTPCH by dual mechanisms. At a relatively low concentration, DAHP emulates BH4 and engages the GFRP-dependent feedback inhibitory system; at higher concentrations, DAHP competes directly for binding with GTP substrate. This knowledge predicts that DAHP would preferably target GTPCH in tissues with abundant GFRP.
Selective inhibitors of neuronal nitric oxide synthase--is no NOS really good NOS for the nervous system?[Pubmed:9226999]
Trends Pharmacol Sci. 1997 Jun;18(6):204-11.
It is now ten years since NO was shown to account for the biological activity of endothelium-derived relaxing factor (EDRF). It is also the tenth anniversary of the identification of L-NG monomethyl arginine (L-NMMA) as the very first inhibitor of NO biosynthesis. That EDRF and NO were one and the same sparked an explosion of interest in the biochemistry and pharmacology of NO which has yet to subside. In contrast, the first ever nitric oxide synthase (NOS) inhibitor slipped seamlessly into the literature virtually without comment at the time. Over the following decade, L-NMMA (and like NOS inhibitors) have proved invaluable as tools for probing the biological roles of NO in health and disease and, in particular, have increased our understanding of the function of NO in the nervous system. Further advances in this important area now require the development of inhibitors selective for the neuronal isoform of NOS (nNOS). Here, Philip Moore and Rachel Handy provide an up-to-date account of the literature regarding the biochemical and pharmacological characterization of NOS inhibitors with particular reference to compounds with greater selectivity for the nNOS isoform.
Pteridine biosynthesis in human endothelial cells. Impact on nitric oxide-mediated formation of cyclic GMP.[Pubmed:7678411]
J Biol Chem. 1993 Jan 25;268(3):1842-6.
Stimulation of nitric oxide (NO) synthase in endothelial cells by Ca2+ influx leads to increased intracellular levels of cGMP. NO synthase from various sources is known to use tetrahydrobiopterin, flavins, and NADPH as cofactors. We studied the effect of interferon-gamma, tumor necrosis factor-alpha, and lipopolysaccharide on tetrahydrobiopterin biosynthetic activities in human umbilical vein endothelial cells (HUVEC). These stimuli led to an up to 40-fold increase of GTP cyclohydrolase I (EC 3.5.4.16) activity and to increased accumulation of neopterin and tetrahydrobiopterin in HUVEC. Further enzyme activities of tetrahydrobiopterin biosynthesis, i.e. 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase (EC 1.1.1.153), remained unchanged. NO synthase activity in protein fractions from homogenates of cells treated with interferon-gamma plus tumor necrosis factor-alpha was not influenced as compared with untreated controls. However, interferon-gamma alone or in combination with tumor necrosis factor-alpha significantly increased intracellular cGMP formation in intact HUVEC by 50 and 80%, respectively. These stimuli increased intracellular tetrahydrobiopterin concentrations up to 14-fold. NO-triggered cGMP formation was similarly increased by incubation of otherwise untreated cells with sepiapterin, leading to elevated intracellular tetrahydrobiopterin levels. Thus, cytokines indirectly stimulate the activity of constitutive NO synthase in HUVEC by upregulating production of the cofactor tetrahydrobiopterin.


