DCC-2036 (Rebastinib)Bcr-Abl inhibitor CAS# 1020172-07-9 |
- Batimastat (BB-94)
Catalog No.:BCC1223
CAS No.:130370-60-4
- CTS-1027
Catalog No.:BCC1502
CAS No.:193022-04-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1020172-07-9 | SDF | Download SDF |
| PubChem ID | 25066467 | Appearance | Powder |
| Formula | C30H28FN7O3 | M.Wt | 553.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Rebastinib | ||
| Solubility | DMSO : 50 mg/mL (90.32 mM; ultrasonic and warming and heat to 80°C) | ||
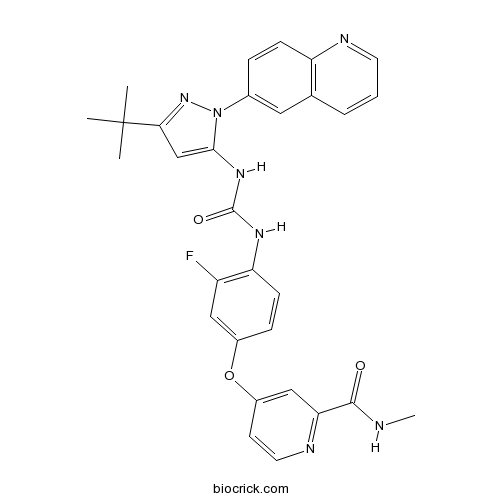
| Chemical Name | 4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)carbamoylamino]-3-fluorophenoxy]-N-methylpyridine-2-carboxamide | ||
| SMILES | CC(C)(C)C1=NN(C(=C1)NC(=O)NC2=C(C=C(C=C2)OC3=CC(=NC=C3)C(=O)NC)F)C4=CC5=C(C=C4)N=CC=C5 | ||
| Standard InChIKey | WVXNSAVVKYZVOE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H28FN7O3/c1-30(2,3)26-17-27(38(37-26)19-7-9-23-18(14-19)6-5-12-33-23)36-29(40)35-24-10-8-20(15-22(24)31)41-21-11-13-34-25(16-21)28(39)32-4/h5-17H,1-4H3,(H,32,39)(H2,35,36,40) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | DCC-2036 (Rebastinib) is an inhibitor of Bcr-Abl with IC50 of 0.8 nM and 4 nM for Abl1(WT) and Abl1(T315I), respectively. | ||||||
| Targets | u-Abl1 (native) | Abl1 (H396P) | p-Abl1 (native) | FLT3 | p-Abl1 (T315I) | ||
| IC50 | 0.75 nM | 1.4 nM | 2 nM | 2 nM | 4 nM | ||

DCC-2036 (Rebastinib) Dilution Calculator

DCC-2036 (Rebastinib) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8064 mL | 9.032 mL | 18.0639 mL | 36.1278 mL | 45.1598 mL |
| 5 mM | 0.3613 mL | 1.8064 mL | 3.6128 mL | 7.2256 mL | 9.032 mL |
| 10 mM | 0.1806 mL | 0.9032 mL | 1.8064 mL | 3.6128 mL | 4.516 mL |
| 50 mM | 0.0361 mL | 0.1806 mL | 0.3613 mL | 0.7226 mL | 0.9032 mL |
| 100 mM | 0.0181 mL | 0.0903 mL | 0.1806 mL | 0.3613 mL | 0.4516 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
DCC-2036 is a conformational control inhibitor of ABL1 with IC50 value of 0.8nM [1].
DCC-2036 is synthesized as a dual-anchoring inhibitor that binds both the switch control pocket E282/R386 pair and the Met318 ATP hinge. It shows a IC50 value of 0.8nM. DCC-2036 also exerts potent inhibition of the gatekeeper mutant ABL1T315I with IC50 value of 4nM. DCC-2036 inhibits ABL1 through forcing the kinase domains into inhibitor-bound, inactive Type II conformations. For the purified ABL1, DCC-2036 strongly inhibits unphosphorylated native ABL1, phosphorylated native ABL1, ABL1H396P, unphosphorylated ABL1T315I and phosphorylated ABL1T315I with IC50 values of 0.82nM, 2nM, 1.4nM, 5nM and 4nM, respectively. It is found that DCC-2036 inhibits ABL1 in a non-ATP-competitive manner. In cellular assay, DCC-2036 inhibits the proliferation of Ba/F3 and K562 cells with IC50 values of 5.4nM and 5.5nM, respectively. Moreover, treatment of DCC-2036 can effectively prolong survival in mice bearing Ba/F3-BCR-ABL1T315I leukemia cells. DCC-2036 is also capable to inhibit BCR-ABL1 in primary leukemic cells from patients with Ph+ leukemia [1].
References:
[1] Chan W W, Wise S C, Kaufman M D, et al. Conformational control inhibition of the BCR-ABL1 tyrosine kinase, including the gatekeeper T315I mutant, by the switch-control inhibitor DCC-2036. Cancer cell, 2011, 19(4): 556-568.
- SGI-1027
Catalog No.:BCC4588
CAS No.:1020149-73-8
- 20,24-Epoxy-24-methoxy-23(24-25)abeo-dammaran-3-one
Catalog No.:BCN1639
CAS No.:1020074-97-8
- Sulfaclozine
Catalog No.:BCC9155
CAS No.:102-65-8
- Phenyethyl 3-methylcaffeate
Catalog No.:BCN8457
CAS No.:71835-85-3
- 3,4-Dihydroxyphenylacetic Acid
Catalog No.:BCC8281
CAS No.:102-32-9
- Acetoacetanilide
Catalog No.:BCC8803
CAS No.:102-01-2
- GW791343 dihydrochloride
Catalog No.:BCC1613
CAS No.:1019779-04-4
- Zardaverine
Catalog No.:BCC2069
CAS No.:101975-10-4
- Octacosyl (E)-ferulate
Catalog No.:BCN5834
CAS No.:101959-37-9
- S0859
Catalog No.:BCC1914
CAS No.:1019331-10-2
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- Regorafenib monohydrate
Catalog No.:BCC1884
CAS No.:1019206-88-2
- (R)-4-Benzyl-2-oxazolidinone
Catalog No.:BCC8395
CAS No.:102029-44-7
- PF-04457845
Catalog No.:BCC1851
CAS No.:1020315-31-4
- Protosappanin A
Catalog No.:BCN7259
CAS No.:102036-28-2
- Protosappanin B
Catalog No.:BCN2281
CAS No.:102036-29-3
- Tubeimoside I
Catalog No.:BCN1089
CAS No.:102040-03-9
- 3-Deazaneplanocin,DZNep
Catalog No.:BCC1129
CAS No.:102052-95-9
- Arctinol B
Catalog No.:BCN5835
CAS No.:102054-39-7
- Sappanone A
Catalog No.:BCN2996
CAS No.:102067-84-5
- Boc-D-Phenylglycinol
Catalog No.:BCC2711
CAS No.:102089-74-7
- 3-(4-Hydroxyphenyl)-1-propanol
Catalog No.:BCN5836
CAS No.:10210-17-0
- Pseudoproto Pb
Catalog No.:BCN2838
CAS No.:102100-46-9
- Pseudoprotodioscin
Catalog No.:BCN2827
CAS No.:102115-79-7
Phase 1 dose-finding study of rebastinib (DCC-2036) in patients with relapsed chronic myeloid leukemia and acute myeloid leukemia.[Pubmed:27927766]
Haematologica. 2017 Mar;102(3):519-528.
A vailable tyrosine kinase inhibitors for chronic myeloid leukemia bind in an adenosine 5'-triphosphate-binding pocket and are affected by evolving mutations that confer resistance. Rebastinib was identified as a switch control inhibitor of BCR-ABL1 and FLT3 and may be active against resistant mutations. A Phase 1, first-in-human, single-agent study investigated rebastinib in relapsed or refractory chronic or acute myeloid leukemia. The primary objectives were to investigate the safety of rebastinib and establish the maximum tolerated dose and recommended Phase 2 dose. Fifty-seven patients received treatment with rebastinib. Sixteen patients were treated using powder-in-capsule preparations at doses from 57 mg to 1200 mg daily, and 41 received tablet preparations at doses of 100 mg to 400 mg daily. Dose-limiting toxicities were dysarthria, muscle weakness, and peripheral neuropathy. The maximum tolerated dose was 150 mg tablets administered twice daily. Rebastinib was rapidly absorbed. Bioavailability was 3- to 4-fold greater with formulated tablets compared to unformulated capsules. Eight complete hematologic responses were achieved in 40 evaluable chronic myeloid leukemia patients, 4 of which had a T315I mutation. None of the 5 patients with acute myeloid leukemia responded. Pharmacodynamic analysis showed inhibition of phosphorylation of substrates of BCR-ABL1 or FLT3 by rebastinib. Although clinical activity was observed, clinical benefit was insufficient to justify continued development in chronic or acute myeloid leukemia. Pharmacodynamic analyses suggest that other kinases inhibited by rebastinib, such as TIE2, may be more relevant targets for the clinical development of rebastinib (clinicaltrials.gov Identifier:00827138).


