Sappanone ACAS# 102067-84-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 102067-84-5 | SDF | Download SDF |
| PubChem ID | 9817274 | Appearance | Cryst. |
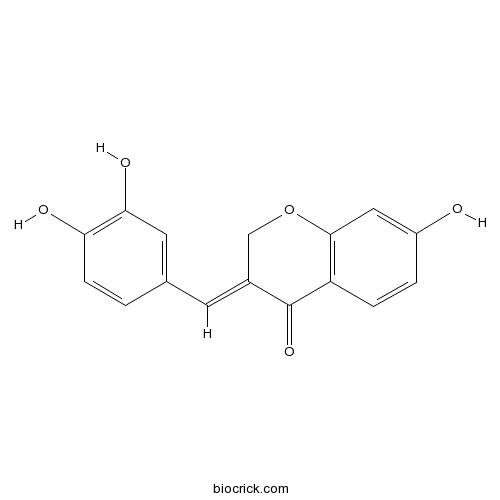
| Formula | C16H12O5 | M.Wt | 284.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3E)-3-[(3,4-dihydroxyphenyl)methylidene]-7-hydroxychromen-4-one | ||
| SMILES | C1C(=CC2=CC(=C(C=C2)O)O)C(=O)C3=C(O1)C=C(C=C3)O | ||
| Standard InChIKey | KVYZXXBTJHJISR-BJMVGYQFSA-N | ||
| Standard InChI | InChI=1S/C16H12O5/c17-11-2-3-12-15(7-11)21-8-10(16(12)20)5-9-1-4-13(18)14(19)6-9/h1-7,17-19H,8H2/b10-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Sappanone A is the first homoisoflavanone to be discovered with melanogenesis inhibitory activity. 2. Sappanone A exerts its anti-inflammatory effect by modulating the Nrf2 and NF-κB pathways, and may be a valuable compound to prevent or treat inflammatory diseases. |
| Targets | NO | PGE | IL Receptor | NOS | COX | Nrf2 | HO-1 | NADPH-oxidase | p38MAPK | p65 | NF-kB |

Sappanone A Dilution Calculator

Sappanone A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5174 mL | 17.5871 mL | 35.1741 mL | 70.3482 mL | 87.9353 mL |
| 5 mM | 0.7035 mL | 3.5174 mL | 7.0348 mL | 14.0696 mL | 17.5871 mL |
| 10 mM | 0.3517 mL | 1.7587 mL | 3.5174 mL | 7.0348 mL | 8.7935 mL |
| 50 mM | 0.0703 mL | 0.3517 mL | 0.7035 mL | 1.407 mL | 1.7587 mL |
| 100 mM | 0.0352 mL | 0.1759 mL | 0.3517 mL | 0.7035 mL | 0.8794 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Arctinol B
Catalog No.:BCN5835
CAS No.:102054-39-7
- 3-Deazaneplanocin,DZNep
Catalog No.:BCC1129
CAS No.:102052-95-9
- Tubeimoside I
Catalog No.:BCN1089
CAS No.:102040-03-9
- Protosappanin B
Catalog No.:BCN2281
CAS No.:102036-29-3
- Protosappanin A
Catalog No.:BCN7259
CAS No.:102036-28-2
- PF-04457845
Catalog No.:BCC1851
CAS No.:1020315-31-4
- (R)-4-Benzyl-2-oxazolidinone
Catalog No.:BCC8395
CAS No.:102029-44-7
- DCC-2036 (Rebastinib)
Catalog No.:BCC4390
CAS No.:1020172-07-9
- SGI-1027
Catalog No.:BCC4588
CAS No.:1020149-73-8
- 20,24-Epoxy-24-methoxy-23(24-25)abeo-dammaran-3-one
Catalog No.:BCN1639
CAS No.:1020074-97-8
- Sulfaclozine
Catalog No.:BCC9155
CAS No.:102-65-8
- Phenyethyl 3-methylcaffeate
Catalog No.:BCN8457
CAS No.:71835-85-3
- Boc-D-Phenylglycinol
Catalog No.:BCC2711
CAS No.:102089-74-7
- 3-(4-Hydroxyphenyl)-1-propanol
Catalog No.:BCN5836
CAS No.:10210-17-0
- Pseudoproto Pb
Catalog No.:BCN2838
CAS No.:102100-46-9
- Pseudoprotodioscin
Catalog No.:BCN2827
CAS No.:102115-79-7
- Cyclocytidine HCl
Catalog No.:BCC5555
CAS No.:10212-25-6
- rac-Rotigotine Hydrochloride
Catalog No.:BCC1881
CAS No.:102120-99-0
- AM580
Catalog No.:BCC5373
CAS No.:102121-60-8
- Atractylic acid dipotassium salt
Catalog No.:BCN5384
CAS No.:102130-43-8
- Isoprocurcumenol
Catalog No.:BCN3528
CAS No.:102130-90-5
- Neoprocurcumenol
Catalog No.:BCN3694
CAS No.:102130-91-6
- RJR 2429 dihydrochloride
Catalog No.:BCC7000
CAS No.:1021418-53-0
- DPCPX
Catalog No.:BCC6649
CAS No.:102146-07-6
Sappanone A exhibits anti-inflammatory effects via modulation of Nrf2 and NF-kappaB.[Pubmed:26122134]
Int Immunopharmacol. 2015 Sep;28(1):328-36.
UNLABELLED: Homoisoflavonoids constitute a small class of natural products. In the present study, we investigated the anti-inflammatory effect of Sappanone A (SPNA), a homoisoflavanone that is isolated from the heartwood of Caesalpinia sappan (Leguminosae), in murine macrophages. SPNA inhibited the production of nitric oxide (NO), prostaglandin E2 (PGE2) and interleukin-6 (IL-6) as well as the expression of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2) and IL-6 in lipopolysaccharide (LPS)-stimulated RAW264.7 cells. Moreover, SPNA protected C57BL/6 mice from LPS-induced mortality. Treatment of RAW264.7 cells with SPNA induced heme oxygenase (HO)-1 protein and mRNA expression and increased nuclear translocation of the nuclear factor-E2-related factor 2 (Nrf2) as well as the expression of Nrf2 target genes such as NAD(P)H: quinone oxidoreductase 1 (NQO1). Knockdown of Nrf2 by siRNA blocked SPNA-mediated HO-1 induction. SB203580, p38 mitogen-activated protein kinase (MAPK) inhibitor, blocked SPNA-induced HO-1 expression and nuclear translocation of Nrf2, suggesting that SPNA induces HO-1 expression by activating Nrf2 through the p38 MAPK pathway. Consistent with the notion that the Nrf2/HO-1 pathway has anti-inflammatory properties, inhibiting HO-1 significantly abrogated the anti-inflammatory effects of SPNA in LPS-stimulated RAW264.7 cells. Moreover, SPNA suppressed LPS-induced nuclear factor kappaB (NF-kappaB) activation via inhibiting Ser 536 phosphorylation and transcriptional activity of RelA/p65 subunit of NF-kappaB. Taken together, these findings suggest that SPNA exerts its anti-inflammatory effect by modulating the Nrf2 and NF-kappaB pathways, and may be a valuable compound to prevent or treat inflammatory diseases.
Melanogenesis inhibition by homoisoflavavone sappanone A from Caesalpinia sappan.[Pubmed:22949866]
Int J Mol Sci. 2012;13(8):10359-67.
Homoisoflavanone, Sappanone A, was isolated from Caesalpinia sappan and proven to dose-dependently inhibit both melanogenesis and cellular tyrosinase activity via repressing tyrosinase gene expression in mouse B16 melanoma cells. To our knowledge, Sappanone A is the first homoisoflavanone to be discovered with melanogenesis inhibitory activity. Our results give a new impetus to the future search for other homoisoflavanone melanogenesis inhibitors.


