DPCPXA1 selective antagonist CAS# 102146-07-6 |
- Batimastat (BB-94)
Catalog No.:BCC1223
CAS No.:130370-60-4
- Batimastat sodium salt
Catalog No.:BCC2075
CAS No.:130464-84-5
- Marimastat
Catalog No.:BCC2118
CAS No.:154039-60-8
- CTS-1027
Catalog No.:BCC1502
CAS No.:193022-04-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 102146-07-6 | SDF | Download SDF |
| PubChem ID | 1329 | Appearance | Powder |
| Formula | C16H24N4O2 | M.Wt | 304.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 5 mM in DMSO and to 10 mM in ethanol | ||
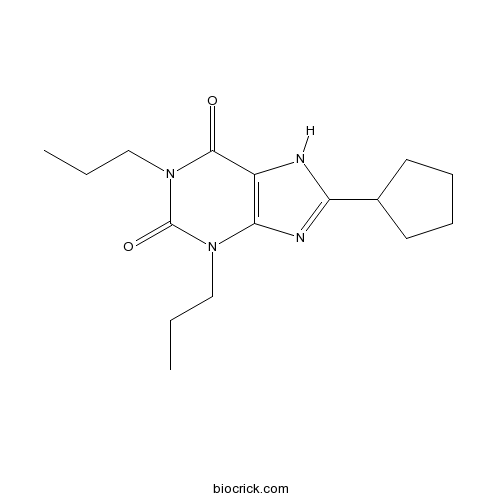
| Chemical Name | 8-cyclopentyl-1,3-dipropyl-7H-purine-2,6-dione | ||
| SMILES | CCCN1C2=C(C(=O)N(C1=O)CCC)NC(=N2)C3CCCC3 | ||
| Standard InChIKey | FFBDFADSZUINTG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective A1 adenosine receptor antagonist, both in vitro and in vivo. Ki values are 3.9, 130, 50 and 4000 nM for human A1, A2A, A2B and A3 receptors respectively. |

DPCPX Dilution Calculator

DPCPX Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2853 mL | 16.4263 mL | 32.8526 mL | 65.7052 mL | 82.1315 mL |
| 5 mM | 0.6571 mL | 3.2853 mL | 6.5705 mL | 13.141 mL | 16.4263 mL |
| 10 mM | 0.3285 mL | 1.6426 mL | 3.2853 mL | 6.5705 mL | 8.2131 mL |
| 50 mM | 0.0657 mL | 0.3285 mL | 0.6571 mL | 1.3141 mL | 1.6426 mL |
| 100 mM | 0.0329 mL | 0.1643 mL | 0.3285 mL | 0.6571 mL | 0.8213 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- RJR 2429 dihydrochloride
Catalog No.:BCC7000
CAS No.:1021418-53-0
- Neoprocurcumenol
Catalog No.:BCN3694
CAS No.:102130-91-6
- Isoprocurcumenol
Catalog No.:BCN3528
CAS No.:102130-90-5
- Atractylic acid dipotassium salt
Catalog No.:BCN5384
CAS No.:102130-43-8
- AM580
Catalog No.:BCC5373
CAS No.:102121-60-8
- rac-Rotigotine Hydrochloride
Catalog No.:BCC1881
CAS No.:102120-99-0
- Cyclocytidine HCl
Catalog No.:BCC5555
CAS No.:10212-25-6
- Pseudoprotodioscin
Catalog No.:BCN2827
CAS No.:102115-79-7
- Pseudoproto Pb
Catalog No.:BCN2838
CAS No.:102100-46-9
- 3-(4-Hydroxyphenyl)-1-propanol
Catalog No.:BCN5836
CAS No.:10210-17-0
- Boc-D-Phenylglycinol
Catalog No.:BCC2711
CAS No.:102089-74-7
- Sappanone A
Catalog No.:BCN2996
CAS No.:102067-84-5
- Boc-D-Pro-OSu
Catalog No.:BCC3438
CAS No.:102185-34-2
- Boc-Arg(Mts)-OH
Catalog No.:BCC3054
CAS No.:102185-38-6
- Boc-D-N-Me-Phe.DCHA
Catalog No.:BCC3347
CAS No.:102185-45-5
- PPNDS
Catalog No.:BCC7015
CAS No.:1021868-77-8
- ARL 67156 trisodium salt
Catalog No.:BCC7004
CAS No.:1021868-83-6
- AGN 192403 hydrochloride
Catalog No.:BCC6924
CAS No.:1021868-90-5
- ZM 39923 HCl
Catalog No.:BCC2203
CAS No.:1021868-92-7
- 3-O-Methyltirotundin
Catalog No.:BCN5837
CAS No.:1021945-29-8
- Boc-4-oxo-Pro-OMe
Catalog No.:BCC3436
CAS No.:102195-80-2
- Tyrosine kinase inhibitor
Catalog No.:BCC2020
CAS No.:1021950-26-4
- ARN2966
Catalog No.:BCC8074
CAS No.:102212-26-0
- Btk inhibitor 1 R enantiomer
Catalog No.:BCC5125
CAS No.:1022150-12-4
Transporter Protein-Coupled DPCPX Nanoconjugates Induce Diaphragmatic Recovery after SCI by Blocking Adenosine A1 Receptors.[Pubmed:27013674]
J Neurosci. 2016 Mar 23;36(12):3441-52.
UNLABELLED: Respiratory complications in patients with spinal cord injury (SCI) are common and have a negative impact on the quality of patients' lives. Systemic administration of drugs that improve respiratory function often cause deleterious side effects. The present study examines the applicability of a novel nanotechnology-based drug delivery system, which induces recovery of diaphragm function after SCI in the adult rat model. We developed a protein-coupled nanoconjugate to selectively deliver by transsynaptic transport small therapeutic amounts of an A1 adenosine receptor antagonist to the respiratory centers. A single administration of the nanoconjugate restored 75% of the respiratory drive at 0.1% of the systemic therapeutic drug dose. The reduction of the systemic dose may obviate the side effects. The recovery lasted for 4 weeks (the longest period studied). These findings have translational implications for patients with respiratory dysfunction after SCI. SIGNIFICANCE STATEMENT: The leading causes of death in humans following SCI are respiratory complications secondary to paralysis of respiratory muscles. Systemic administration of methylxantines improves respiratory function but also leads to the development of deleterious side effects due to actions of the drug on nonrespiratory sites. The importance of the present study lies in the novel drug delivery approach that uses nanotechnology to selectively deliver recovery-inducing drugs to the respiratory centers exclusively. This strategy allows for a reduction in the therapeutic drug dose, which may reduce harmful side effects and markedly improve the quality of life for SCI patients.
ZM241385, DPCPX, MRS1706 are inverse agonists with different relative intrinsic efficacies on constitutively active mutants of the human adenosine A2B receptor.[Pubmed:17077318]
J Pharmacol Exp Ther. 2007 Feb;320(2):637-45.
The human adenosine A(2B) receptor belongs to class A G protein-coupled receptors (GPCRs). In our previous work, constitutively active mutant (CAM) human adenosine A(2B) receptors were identified from a random mutation bank. In the current study, three known A(2B) receptor antagonists, 4-{2-[7-amino-2-(2-furyl)[1,2,4]triazolo-[2,3-a][1,3,5]triazin-5-yl-amino]ethyl}p henol (ZM241385), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), and N-(4-acetylphenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl) phenoxy]acetamide (MRS1706) were tested on wild-type and nine CAM A(2B) receptors with different levels of constitutive activity in a yeast growth assay. All three compounds turned out to be inverse agonists for the adenosine A(2B) receptor because they were able to fully reverse the basal activity of four low-level constitutively active A(2B) receptor mutants and to partially reverse the basal activity of three medium-level constitutively active A(2B) receptor mutants. We also discovered two highly constitutively active mutants whose basal activity could not be reversed by any of the three compounds. A two-state receptor model was used to explain the experimental observations; fitting these yielded the following relative intrinsic efficacies for the three inverse agonists ZM241385, DPCPX, and MRS1706: 0.14 +/- 0.03, 0.35 +/- 0.03, and 0.31 +/- 0.02, respectively. Moreover, varying L, the ratio of active versus inactive receptors in this model, from 0.11 for mutant F84L to 999 for two highly constitutively active mutants yielded simulated dose-response curves that mimicked the experimental curves. This study is the first description of inverse agonists for the human adenosine A(2B) receptor. Moreover, the use of receptor mutants with varying levels of constitutive activity enabled us to determine the relative intrinsic efficacy of these inverse agonists.
DPCPX-resistant hypoxic synaptic depression in the CA1 region of hippocampal slices: possible role of intracellular accumulation of monocarboxylates.[Pubmed:16714083]
Neurosci Lett. 2006 Jul 31;403(1-2):141-6.
Adenosine plays the principal role in synaptic depression during various energy-depleted conditions. However, additional inhibitory factors not associated with A1 adenosine receptors appear to be involved in hypoxic insults. Monocarboxylate accumulation and consequent acidic changes during hypoxia may be responsible for this remaining depression in synaptic activity. Field evoked potentials were recorded in the CA1 region of rat hippocampal slices. Preincubation with 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) disclosed 43% of DPCPX-resistant synaptic depression (DRSD) during oxygen deprivation (OD). In contrast, no DRSD was detected in various conditions with limited glucose utilization, such as glucose deprivation and oxygen-glucose deprivation. Inhibition of anaerobic glycolysis (iodoacetate, sodium fluoride) abolished DRSD during OD, whereas blockade of monocarboxylate utilization with alpha-cyano-4-hydroxycinnamic acid (4-CIN) provoked DRSD in normoxic medium. These observations suggest that an intracellular accumulation of monocarboxylates is responsible for DRSD during hypoxia.
Oral tremor induced by the muscarinic agonist pilocarpine is suppressed by the adenosine A2A antagonists MSX-3 and SCH58261, but not the adenosine A1 antagonist DPCPX.[Pubmed:19958787]
Pharmacol Biochem Behav. 2010 Feb;94(4):561-9.
Tremulous jaw movements in rats, which can be induced by dopamine (DA) antagonists, DA depletion, and cholinomimetics, have served as a useful model for studies of tremor. Although adenosine A(2A) antagonists can reduce the tremulous jaw movements induced by DA antagonists and DA depletion, there are conflicting reports about the interaction between adenosine antagonists and cholinomimetic drugs. The present studies investigated the ability of adenosine antagonists to reverse the tremorogenic effect of the muscarinic agonist pilocarpine. While the adenosine A(2A) antagonist MSX-3 was incapable of reversing the tremulous jaw movements induced by the 4.0mg/kg dose of pilocarpine, both MSX-3 and the adenosine A(2A) antagonist SCH58261 reversed the tremulous jaw movements elicited by 0.5mg/kg pilocarpine. Systemic administration of the adenosine A(1) antagonist DPCPX failed to reverse the tremulous jaw movements induced by either an acute 0.5mg/kg dose of the cholinomimetic pilocarpine or the DA D2 antagonist pimozide, indicating that the tremorolytic effects of adenosine antagonists may be receptor subtype specific. Behaviorally active doses of MSX-3 and SCH 58261 showed substantial in vivo occupancy of A(2A) receptors, but DPCPX did not. The results of these studies support the use of adenosine A(2A) antagonists for the treatment of tremor.
An experimental paradigm for investigating the role of endogenous adenosine/A1 receptor interactions in vivo.[Pubmed:1432696]
J Pharmacol Exp Ther. 1992 Nov;263(2):657-62.
The purpose of the present study was to develop a pharmacological method for determining in the rat in vivo whether endogenous adenosine participates in a given process via activation of A1 adenosine receptors. In anesthetized rats, A1 receptors were activated by infusing the highly selective A1 receptor agonist N6-cyclopentyladenosine, and A2 receptors were stimulated by infusing the highly selective A2 receptor agonist CGS21680C. The bradycardic response to N6-cyclopentyladenosine and the hypotensive response to CGS21680C were used to assess A1 receptor and A2 receptor activation, respectively. After control responses to these purinergic agonists were elicited, animals were given infusions for several hours of either vehicle or one of six dosage levels of FK453 (a potent, selective, nonxanthine A1 receptor antagonist), one of three dosage levels of FR113452 (the S-enantiomer of FK453) or one of seven dosage levels of DPCPX (a potent, selective, xanthine A1 receptor antagonist). Antagonists were infused for > 4 hr, and at various times during the infusions, bradycardic and hypotensive responses to N6-cyclopentyladenosine and CGS21680C, respectively, were reassessed. Both FK453 and DPCPX were highly potent A1 receptor antagonists in vivo, and complete inhibition of bradycardic responses to N6-cyclopentyladenosine were obtained with 3 and 1 micrograms/kg/min, respectively. FR113452 was a very weak antagonist and only slightly reduced bradycardic responses to N6-cyclopentyladenosine at 100 micrograms/kg/min. In vivo FK453 and DPCPX were > 300 and 1000 times selective for the A1 receptor, respectively, compared with the A2 receptor.(ABSTRACT TRUNCATED AT 250 WORDS)
Effects of 1,3-dipropyl-8-cyclopentylxanthine (DPCPX), a highly selective adenosine receptor antagonist, on force of contraction in guinea-pig atrial and ventricular cardiac preparations.[Pubmed:2554151]
Naunyn Schmiedebergs Arch Pharmacol. 1989 Aug;340(2):204-9.
The effects of the A1 adenosine receptor antagonist 1,3-dipropyl-8-cyclopentylxanthine (DPCPX) on force of contraction were examined in isolated electrically driven auricles and papillary muscles from guinea-pigs in the absence and presence of (-)-N6-phenylisopropyladenosine (PIA) and 5'-N-ethylcarboxamidadenosine (NECA). In auricles DPCPX (30-1000 mmol/l) alone increased force of contraction. DPCPX produced only a minor inhibition of phosphodiesterase I-III activity. PIA and NECA alone exerted concentration-dependent negative inotropic effects and the concentration-response curves for PIA and NECA were shifted competitively to the right by the adenosine receptor antagonist DPCPX with similar potency and efficacy. The pA2-value for the inhibition of the effects of PIA and NECA were 9.1 and 8.8, respectively. In papillary muscles DPCPX alone had no inotropic effect. In the presence of isoprenaline PIA and NECA alone exerted concentration-dependent negative inotropic effects and again DPCPX shifted the concentration-response curves for PIA and NECA competitively to the right with similar potency and efficacy. The pA2-value for the inhibition of the effects of PIA and NECA were 9.3 and 9.0, respectively. It is concluded that DPCPX is a potent competitive A1 adenosine receptor antagonist in guinea-pig atrial and ventricular cardiac preparations. Since PIA and NECA were equally potent the cardiac adenosine receptor may constitute a subtype of A1 adenosine receptors differing from the receptor in other tissues such as fat cells. Furthermore, DPCPX has a positive inotropic effect in atrial tissue which cannot be attributed to the A1 receptor antagonism.


