Protosappanin BCAS# 102036-29-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 102036-29-3 | SDF | Download SDF |
| PubChem ID | 13846690 | Appearance | Powder |
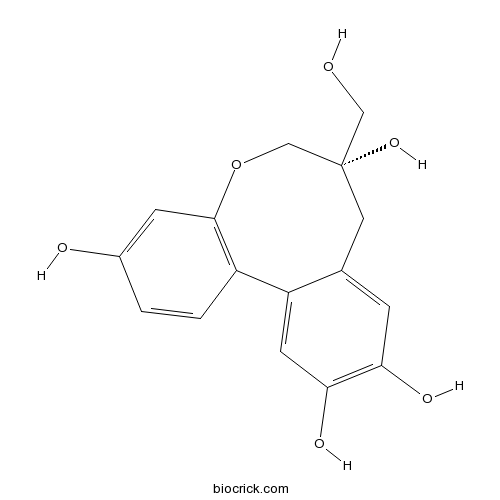
| Formula | C16H16O6 | M.Wt | 304.30 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | DMSO : 250 mg/mL (821.58 mM; Need ultrasonic) | ||
| SMILES | C1C2=CC(=C(C=C2C3=C(C=C(C=C3)O)OCC1(CO)O)O)O | ||
| Standard InChIKey | QRTYTQTVJQUCEP-INIZCTEOSA-N | ||
| Standard InChI | InChI=1S/C16H16O6/c17-7-16(21)6-9-3-13(19)14(20)5-12(9)11-2-1-10(18)4-15(11)22-8-16/h1-5,17-21H,6-8H2/t16-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Protosappanin B possesses antitumor, anti-inflammation and anti-oxidation properties, it protects PC12 cells against oxygen–glucose deprivation-induced neuronal death by maintaining mitochondrial homeostasis via induction of ubiquitin-dependent p53 protein degradation. |
| Targets | MMP(e.g.TIMP) | p53 | Caspase | Bcl-2/Bax | NOS | NO |
| In vitro | Antitumor Effects of Purified Protosappanin B Extracted From Lignum Sappan.[Pubmed: 26036624 ]Integr Cancer Ther. 2016 Mar;15(1):87-95.To assess the antitumor effects of Protosappanin B extracted from Lignum Sappan.
|
| In vivo | An LC/MS/MS method for simultaneous quantitation of two homoisoflavones: protosappanin B and brazilin with hypoglycemic activity in rat plasma and its application to a comparative pharmacokinetic study in normal and streptozotocin-treated rats.[Pubmed: 23707335]J Ethnopharmacol. 2013 Jul 9;148(2):682-90.The heartwood of Caesalpinia sappan L. (Leguminosae), a widely used Chinese medicine in folk, has been used for the treatment of traumatic injury, stasis pain, amenorrhea, dysmenorrheal, as well as stabbing pain in the chest, abdomen and so on. Protosappanin B and brazilin, as the major bioactive homoisoflavones of Sappan Lignum, are used as the marker components for the quality control of the herb in China Pharmacopoeia. To establish a sensitive LC/MS/MS method for investigating the pharmacokinetic properties of Protosappanin B and brazilin in rats after oral administration of Sappan Lignum extract, and compare their pharmacokinetics difference between normal and streptozotocin-treated rats. |
| Kinase Assay | In vitro study for inhibition of NO production about constituents of Sappan Lignum.[Pubmed: 17202686]Protosappanin B protects PC12 cells against oxygen-glucose deprivation-induced neuronal death by maintaining mitochondrial homeostasis via induction of ubiquitin-dependent p53 protein degradation.[Pubmed: 25657114 ]Eur J Pharmacol. 2015 Mar 15;751:13-23.Protosappanin B (PTB) is a bioactive dibenzoxocin derivative isolated from Caesalpinia sappan L. Biol Pharm Bull. 2007 Jan;30(1):193-6.
|

Protosappanin B Dilution Calculator

Protosappanin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2862 mL | 16.4312 mL | 32.8623 mL | 65.7246 mL | 82.1558 mL |
| 5 mM | 0.6572 mL | 3.2862 mL | 6.5725 mL | 13.1449 mL | 16.4312 mL |
| 10 mM | 0.3286 mL | 1.6431 mL | 3.2862 mL | 6.5725 mL | 8.2156 mL |
| 50 mM | 0.0657 mL | 0.3286 mL | 0.6572 mL | 1.3145 mL | 1.6431 mL |
| 100 mM | 0.0329 mL | 0.1643 mL | 0.3286 mL | 0.6572 mL | 0.8216 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Protosappanin A
Catalog No.:BCN7259
CAS No.:102036-28-2
- PF-04457845
Catalog No.:BCC1851
CAS No.:1020315-31-4
- (R)-4-Benzyl-2-oxazolidinone
Catalog No.:BCC8395
CAS No.:102029-44-7
- DCC-2036 (Rebastinib)
Catalog No.:BCC4390
CAS No.:1020172-07-9
- SGI-1027
Catalog No.:BCC4588
CAS No.:1020149-73-8
- 20,24-Epoxy-24-methoxy-23(24-25)abeo-dammaran-3-one
Catalog No.:BCN1639
CAS No.:1020074-97-8
- Sulfaclozine
Catalog No.:BCC9155
CAS No.:102-65-8
- Phenyethyl 3-methylcaffeate
Catalog No.:BCN8457
CAS No.:71835-85-3
- 3,4-Dihydroxyphenylacetic Acid
Catalog No.:BCC8281
CAS No.:102-32-9
- Acetoacetanilide
Catalog No.:BCC8803
CAS No.:102-01-2
- GW791343 dihydrochloride
Catalog No.:BCC1613
CAS No.:1019779-04-4
- Zardaverine
Catalog No.:BCC2069
CAS No.:101975-10-4
- Tubeimoside I
Catalog No.:BCN1089
CAS No.:102040-03-9
- 3-Deazaneplanocin,DZNep
Catalog No.:BCC1129
CAS No.:102052-95-9
- Arctinol B
Catalog No.:BCN5835
CAS No.:102054-39-7
- Sappanone A
Catalog No.:BCN2996
CAS No.:102067-84-5
- Boc-D-Phenylglycinol
Catalog No.:BCC2711
CAS No.:102089-74-7
- 3-(4-Hydroxyphenyl)-1-propanol
Catalog No.:BCN5836
CAS No.:10210-17-0
- Pseudoproto Pb
Catalog No.:BCN2838
CAS No.:102100-46-9
- Pseudoprotodioscin
Catalog No.:BCN2827
CAS No.:102115-79-7
- Cyclocytidine HCl
Catalog No.:BCC5555
CAS No.:10212-25-6
- rac-Rotigotine Hydrochloride
Catalog No.:BCC1881
CAS No.:102120-99-0
- AM580
Catalog No.:BCC5373
CAS No.:102121-60-8
- Atractylic acid dipotassium salt
Catalog No.:BCN5384
CAS No.:102130-43-8
An LC/MS/MS method for simultaneous quantitation of two homoisoflavones: protosappanin B and brazilin with hypoglycemic activity in rat plasma and its application to a comparative pharmacokinetic study in normal and streptozotocin-treated rats.[Pubmed:23707335]
J Ethnopharmacol. 2013 Jul 9;148(2):682-90.
ETHNOPHARMACOLOGICAL RELEVANCE: The heartwood of Caesalpinia sappan L. (Leguminosae), a widely used Chinese medicine in folk, has been used for the treatment of traumatic injury, stasis pain, amenorrhea, dysmenorrheal, as well as stabbing pain in the chest, abdomen and so on. Protosappanin B and brazilin, as the major bioactive homoisoflavones of Sappan Lignum, are used as the marker components for the quality control of the herb in China Pharmacopoeia. AIM OF THE STUDY: To establish a sensitive LC/MS/MS method for investigating the pharmacokinetic properties of Protosappanin B and brazilin in rats after oral administration of Sappan Lignum extract, and compare their pharmacokinetics difference between normal and streptozotocin-treated rats. MATERIAL AND METHODS: A rapid, selective and sensitive LC/MS/MS method was developed and validated for the simultaneous quantification of Protosappanin B and brazilin in rat plasma. Normal and streptozotocin-treated rats were orally administered with the Sappan Lignum extract at the same dose of 2.83 g extract/kg body weight (equivalent to 35.56 mg/kg of Protosappanin B and 52.25 mg/kg of brazilin), respectively. RESULTS: After oral administration of Sappan Lignum extract, a remarkable increase (p<0.05) in the value of AUC0-24h, AUC0-infinity, Cmax and T1/2 associated with Protosappanin B and brazilin was observed in the streptozotocin-treated group. Compared with the normal rats, elimination of both compounds in the streptozotocin-treated rats was slower. CONCLUSION: The established method was successfully applied to compare the pharmacokinetic behaviors of Protosappanin B and brazilin in rat plasma after oral administration of Sappan Lignum extract between normal and streptozotocin-treated groups; the results might suggest the accumulation of both compounds in diabetic pathologic states and the adverse reaction should be considered when it was used.
Antitumor Effects of Purified Protosappanin B Extracted From Lignum Sappan.[Pubmed:26036624]
Integr Cancer Ther. 2016 Mar;15(1):87-95.
HYPOTHESIS: To assess the antitumor effects of Protosappanin B extracted from Lignum Sappan. STUDY DESIGN: Lignum Sappan was sequentially extracted by boiling water and ethyl acetate. The resulting extract was separated by column chromatography, to yield Protosappanin B. The compound was then identified by thin-layer chromatography, high-performance liquid chromatography, elemental analysis, and spectrometry (infrared and ultraviolet). The effects on tumor cell viability and growth of purified Protosappanin B were evaluated in vitro by trypan blue exclusion and MTT assays, respectively. And the effects of Protosappanin B were assessed in vivo, on H22 mouse liver cancer cell invasion and the survival of tumor-bearing mice. RESULTS: Protosappanin B (2 mg/mL) reduced the viability of human bladder cancer T24 cells and mouse bladder cancer BTT cells in a time-dependent manner (P < .05) and significantly inhibited the growth of the human colon cancer cell lines HCT-116 and SW-480. IC50 values of 21.32, 26.73, and 76.53 microg/mL were obtained for SW-480, HCT-116, and BTT cells, respectively, after 48 hours of treatment with Protosappanin B. In addition, pretreatment of H22 cells with Protosappanin B (final concentration = 6.25 mg/mL) resulted in complete inhibition of tumor formation in KM mice. Furthermore, Protosappanin B (200 and 300 mg/kg) significantly increased the survival of BTT tumor-bearing T739 mice, at a rate comparable to that of 1 mg/kg mitomycin. CONCLUSION: Protosappanin B extracted from Lignum Sappan exerts marked antitumor effects both in vitro and in vivo.
Protosappanin B protects PC12 cells against oxygen-glucose deprivation-induced neuronal death by maintaining mitochondrial homeostasis via induction of ubiquitin-dependent p53 protein degradation.[Pubmed:25657114]
Eur J Pharmacol. 2015 Mar 15;751:13-23.
Protosappanin B (PTB) is a bioactive dibenzoxocin derivative isolated from Caesalpinia sappan L. Here, we investigated the neuroprotective effects and the potential mechanisms of PTB on oxygen-glucose deprivation (OGD)-injured PC12 cells. Results showed that PTB significantly increased cell viability, inhibited cell apoptosis and up-regulated the expression of growth-associated protein 43 (a marker of neural outgrowth). Moreover, our study revealed that PTB effectively maintained mitochondrial homeostasis by up-regulation of mitochondrial membrane potential (MMP), inhibition of cytochrome c release from mitochondria and inactivation of mitochondrial caspase-9/3 apoptosis pathway. Further study showed that PTB significantly promoted cytoplasmic component degradation of p53 protein, a key negative regulator for mitochondrial function, resulting in a release of Bcl-2 from p53-Bcl-2 complex and an enhancing translocation of Bcl-2 to mitochondrial outer membrane. Finally, we found the degradation of p53 protein was induced by PTB via activation of a MDM2-dependent ubiquitination process. Taken together, our findings provided a new viewpoint of neuronal protection strategy for anoxia and ischemic injury with natural small molecular dibenzoxocin derivative by activating ubiquitin-dependent p53 protein degradation as well as increasing mitochondrial function.
In vitro study for inhibition of NO production about constituents of Sappan Lignum.[Pubmed:17202686]
Biol Pharm Bull. 2007 Jan;30(1):193-6.
In the course of our screening, we found that the methanolic extract of Sappan Lignum showed strong activity against lipopolysaccharide (LPS)-induced nitric oxide (NO) production by macrophages in vitro. As it was reported that Brazilin inhibited inducible NO gene, we conducted to similar tests for six known compounds isolated from Sappan Lignum, namely, brazilein, sappanchalcone, protosappanin A, Protosappanin B, protosappanin C besides brazilin. And six compounds were also subjected to six tests to speculate their properties: (1) inhibition of NO production by cultured J774.1 (macrophage-like) cell line, (2) suppression of inducible NO synthase (iNOS) gene expression, (3) inhibition of NO production by murine peritoneal macrophages, (4) DPPH radical scavenging activity, (5) reduction of ferric ion and (6) antioxidant activity. Brazilein and sappanchalcone showed significant inhibition of lipopolysaccharide (LPS)-induced NO production by J774.1 cell line like Brazilin; 100% inhibition at 30 microM in test (1) and at 10 microM in test (3). The mechanisms underlying the inhibition of NO production by the compounds were investigated in test (2). As a result, brazilin was found to almost completely suppress iNOS gene expression at 100 microM as reported, and brazilein and sappanchalcone also suppressed iNOS gene expression. But strong activities were not observed for protosappanins A, B and C. So, we conducted tests (4), (5) and (6) to investigate other properties about six compounds. Protosappanin A and Brazilin demonstrated high antioxidant activity compared with Vitamin E in tests (4) and (5). Protosappanin A and B inhibited the oxidation of linoleic acid in test (6). Among the dibenzoxocin derivatives, only protosappanin C did not show significant activity in all the tests. We found that sappanchalcone showed same activity as brazilin, and six compounds isolated from Sappan Lignum showed various properties.


