SGI-1027DNMT inhibitor CAS# 1020149-73-8 |
- 3-Deazaneplanocin,DZNep
Catalog No.:BCC1129
CAS No.:102052-95-9
- EPZ005687
Catalog No.:BCC2219
CAS No.:1396772-26-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1020149-73-8 | SDF | Download SDF |
| PubChem ID | 24858111 | Appearance | Powder |
| Formula | C27H23N7O | M.Wt | 461.52 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 27 mg/mL (58.50 mM; Need ultrasonic and warming) H2O : < 0.1 mg/mL (insoluble) | ||
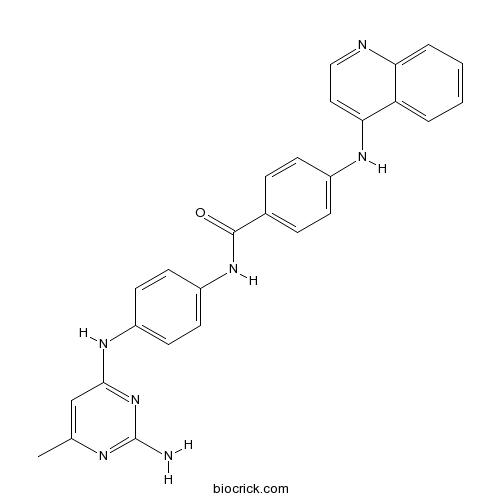
| Chemical Name | N-[4-[(2-amino-6-methylpyrimidin-4-yl)amino]phenyl]-4-(quinolin-4-ylamino)benzamide | ||
| SMILES | CC1=CC(=NC(=N1)N)NC2=CC=C(C=C2)NC(=O)C3=CC=C(C=C3)NC4=CC=NC5=CC=CC=C54 | ||
| Standard InChIKey | QSYLKMKIVWJAAK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H23N7O/c1-17-16-25(34-27(28)30-17)32-20-10-12-21(13-11-20)33-26(35)18-6-8-19(9-7-18)31-24-14-15-29-23-5-3-2-4-22(23)24/h2-16H,1H3,(H,29,31)(H,33,35)(H3,28,30,32,34) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | SGI-1027 is an inhibitor of DNMT with IC50 values of 6, 8, 7.5 μM for DNMT1, DNMT3A, and DNMT3B, respectively | |||||
| Targets | DNMT1 | DNMT3B | DNMT3A | |||
| IC50 | 6 μM | 7.5 μM | 8 μM | |||

SGI-1027 Dilution Calculator

SGI-1027 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1668 mL | 10.8338 mL | 21.6675 mL | 43.3351 mL | 54.1688 mL |
| 5 mM | 0.4334 mL | 2.1668 mL | 4.3335 mL | 8.667 mL | 10.8338 mL |
| 10 mM | 0.2167 mL | 1.0834 mL | 2.1668 mL | 4.3335 mL | 5.4169 mL |
| 50 mM | 0.0433 mL | 0.2167 mL | 0.4334 mL | 0.8667 mL | 1.0834 mL |
| 100 mM | 0.0217 mL | 0.1083 mL | 0.2167 mL | 0.4334 mL | 0.5417 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SGI-1027 is an inhibitor of DNMT with IC50 values of 12.5μM, 8μM and 7.5μM, respectively for DNMT1, DNMT3A and DNMT3B [1].
SGI-1027 shows inhibition with mammalian DNMTs and bacterial M. SssI in vitro. Both the endogenous and recombinant DNMTs can be inhibited by SGI-1027. The mechanism of this inhibition is that SGI-1027 competes with Ado-Met but not the substrate DNA within the cofactor binding site of the enzyme. SGI-1027 inhibits DNA methylation through directly inhibiting DNMTs [1].
In cancer cells, many TSGs are silenced due to hypermethylation of CpG islands in their promoters. SGI-1027 can demethylates these CpG islands and reactivate the silenced TSGs. In RKO cells, prolonged treatment of SGI-1027 induces reexpression of P16 and TIMP3 genes. Moreover, SGI-1027 is found to cause selective degradation of DNMT1 via proteasomal pathway [1].
References:
[1] Datta J, Ghoshal K, Denny WA, Gamage SA, Brooke DG, Phiasivongsa P, Redkar S, Jacob ST. A new class of quinoline-based DNA hypomethylating agents reactivates tumor suppressor genes by blocking DNA methyltransferase 1 activity and inducing its degradation. Cancer Res. 2009 May 15;69(10):4277-85.
- 20,24-Epoxy-24-methoxy-23(24-25)abeo-dammaran-3-one
Catalog No.:BCN1639
CAS No.:1020074-97-8
- Sulfaclozine
Catalog No.:BCC9155
CAS No.:102-65-8
- Phenyethyl 3-methylcaffeate
Catalog No.:BCN8457
CAS No.:71835-85-3
- 3,4-Dihydroxyphenylacetic Acid
Catalog No.:BCC8281
CAS No.:102-32-9
- Acetoacetanilide
Catalog No.:BCC8803
CAS No.:102-01-2
- GW791343 dihydrochloride
Catalog No.:BCC1613
CAS No.:1019779-04-4
- Zardaverine
Catalog No.:BCC2069
CAS No.:101975-10-4
- Octacosyl (E)-ferulate
Catalog No.:BCN5834
CAS No.:101959-37-9
- S0859
Catalog No.:BCC1914
CAS No.:1019331-10-2
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- Regorafenib monohydrate
Catalog No.:BCC1884
CAS No.:1019206-88-2
- Dabigatran etexilate benzenesulfonate
Catalog No.:BCC8925
CAS No.:1019206-65-5
- DCC-2036 (Rebastinib)
Catalog No.:BCC4390
CAS No.:1020172-07-9
- (R)-4-Benzyl-2-oxazolidinone
Catalog No.:BCC8395
CAS No.:102029-44-7
- PF-04457845
Catalog No.:BCC1851
CAS No.:1020315-31-4
- Protosappanin A
Catalog No.:BCN7259
CAS No.:102036-28-2
- Protosappanin B
Catalog No.:BCN2281
CAS No.:102036-29-3
- Tubeimoside I
Catalog No.:BCN1089
CAS No.:102040-03-9
- 3-Deazaneplanocin,DZNep
Catalog No.:BCC1129
CAS No.:102052-95-9
- Arctinol B
Catalog No.:BCN5835
CAS No.:102054-39-7
- Sappanone A
Catalog No.:BCN2996
CAS No.:102067-84-5
- Boc-D-Phenylglycinol
Catalog No.:BCC2711
CAS No.:102089-74-7
- 3-(4-Hydroxyphenyl)-1-propanol
Catalog No.:BCN5836
CAS No.:10210-17-0
- Pseudoproto Pb
Catalog No.:BCN2838
CAS No.:102100-46-9
Synthetic approaches to DNMT inhibitor SGI-1027 and effects on the U937 leukemia cell line.[Pubmed:23402879]
Bioorg Med Chem Lett. 2013 Mar 15;23(6):1631-5.
The known DNMT inhibitor SGI-1027 4 has been synthesized using as key steps Pd-catalyzed Ar-N bond formation reactions performed in a sequential or convergent manner. In the former approach, a by-product, which corresponds to the incorporation of two units of 4-chloroquinoline, was also isolated. The biological effects of compound 4 in the U937 human leukemia cell line are also described.
Molecular modeling studies of the novel inhibitors of DNA methyltransferases SGI-1027 and CBC12: implications for the mechanism of inhibition of DNMTs.[Pubmed:23637988]
PLoS One. 2013 Apr 25;8(4):e62152.
DNA methylation is an epigenetic modification that regulates gene expression by DNA methyltransferases (DNMTs). Inhibition of DNMTs is a promising approach for cancer therapy. Recently, novel classes of the quinolone-based compound, SGI-1027, and RG108-procainamide conjugates, CBC12, have been identified as potent DNMT inhibitors. In this work, we report comprehensive studies using induced-fit docking of SGI-1027 and CBC12 with human DNMT1 and DNMT3A. The docking was performed in the C-terminal MTase catalytic domain, which contains the substrate and cofactor binding sites, in the presence and absence of other domains. Induced-fit docking predicts possible binding modes of the ligands through the appropriate structural changes in the receptor. This work suggests a hypothesis of the inhibitory mechanisms of the new inhibitors which is in agreement with the reported autoinhibitory mechanism. The insights obtained in this work can be used to design DNMT inhibitors with novel scaffolds.
Design, synthesis and biological evaluation of 4-amino-N- (4-aminophenyl)benzamide analogues of quinoline-based SGI-1027 as inhibitors of DNA methylation.[Pubmed:24678024]
ChemMedChem. 2014 Mar;9(3):590-601.
Quinoline derivative SGI-1027 (N-(4-(2-amino-6-methylpyrimidin-4-ylamino)phenyl)-4-(quinolin-4-ylamino)benzamid e) was first described in 2009 as a potent inhibitor of DNA methyltransferase (DNMT) 1, 3A and 3B. Based on molecular modeling studies, performed using the crystal structure of Haemophilus haemolyticus cytosine-5 DNA methyltransferase (MHhaI C5 DNMT), which suggested that the quinoline and the aminopyridimine moieties of SGI-1027 are important for interaction with the substrates and protein, we designed and synthesized 25 derivatives. Among them, four compounds-namely the derivatives 12, 16, 31 and 32-exhibited activities comparable to that of the parent compound. Further evaluation revealed that these compounds were more potent against human DNMT3A than against human DNMT1 and induced the re-expression of a reporter gene, controlled by a methylated cytomegalovirus (CMV) promoter, in leukemia KG-1 cells. These compounds possessed cytotoxicity against leukemia KG-1 cells in the micromolar range, comparable with the cytotoxicity of the reference compound, SGI-1027. Structure-activity relationships were elucidated from the results. First, the presence of a methylene or carbonyl group to conjugate the quinoline moiety decreased the activity. Second, the size and nature of the aromatic or heterocycle subsitutents effects inhibition activity: tricyclic moieties, such as acridine, were found to decrease activity, while bicyclic substituents, such as quinoline, were well tolerated. The best combination was found to be a bicyclic substituent on one side of the compound, and a one-ring moiety on the other side. Finally, the orientation of the central amide bond was found to have little effect on the biological activity. This study provides new insights in to the structure-activity relationships of SGI-1027 and its derivative.


