PF-3845FAAH ihibitor,highly potent and selective CAS# 1196109-52-0 |
- PF-04457845
Catalog No.:BCC1851
CAS No.:1020315-31-4
- FAAH inhibitor 1
Catalog No.:BCC4254
CAS No.:326866-17-5
- JNJ-1661010
Catalog No.:BCC2315
CAS No.:681136-29-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1196109-52-0 | SDF | Download SDF |
| PubChem ID | 25154867 | Appearance | Powder |
| Formula | C24H23F3N4O2 | M.Wt | 456.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (219.08 mM) *"≥" means soluble, but saturation unknown. | ||
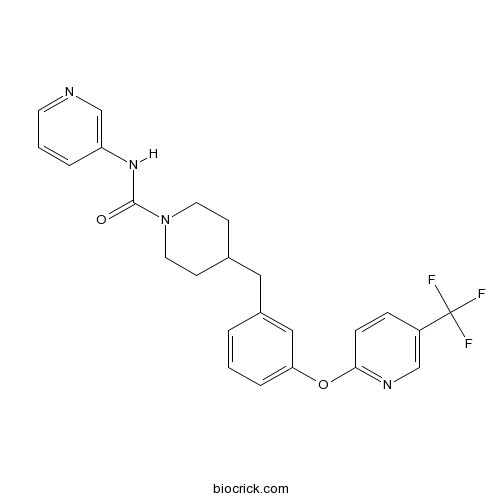
| Chemical Name | N-pyridin-3-yl-4-[[3-[5-(trifluoromethyl)pyridin-2-yl]oxyphenyl]methyl]piperidine-1-carboxamide | ||
| SMILES | C1CN(CCC1CC2=CC(=CC=C2)OC3=NC=C(C=C3)C(F)(F)F)C(=O)NC4=CN=CC=C4 | ||
| Standard InChIKey | NBOJHRYUGLRASX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H23F3N4O2/c25-24(26,27)19-6-7-22(29-15-19)33-21-5-1-3-18(14-21)13-17-8-11-31(12-9-17)23(32)30-20-4-2-10-28-16-20/h1-7,10,14-17H,8-9,11-13H2,(H,30,32) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective fatty acid amide hydrolase (FAAH) inhibitor (Ki = 0.23 μM). Reduces inflammatory pain via a cannabinoid receptor-dependent mechanism. Highly efficacious and selective in vivo. Displays no activity at FAAH-2 (IC50 >10 μM). |

PF-3845 Dilution Calculator

PF-3845 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1908 mL | 10.9539 mL | 21.9077 mL | 43.8154 mL | 54.7693 mL |
| 5 mM | 0.4382 mL | 2.1908 mL | 4.3815 mL | 8.7631 mL | 10.9539 mL |
| 10 mM | 0.2191 mL | 1.0954 mL | 2.1908 mL | 4.3815 mL | 5.4769 mL |
| 50 mM | 0.0438 mL | 0.2191 mL | 0.4382 mL | 0.8763 mL | 1.0954 mL |
| 100 mM | 0.0219 mL | 0.1095 mL | 0.2191 mL | 0.4382 mL | 0.5477 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PF-3845 is a highly potent and selective inhibitor of fatty acid amide hydrolase (FAAH) with Ki value of 0.23μM [1].
PF-3845 is a biaryl ether piperidine. It inhibits FAAH by a covalent, irreversible mechanism involving carbamylating FAAH's catalytic S241 nucleophile. It is found that, administration of PF-3845 to mice results a rapid and complete inactivation of FAAH in the brain. PF-3845 is highly selective for FAAH in vivo. It shows no activity to some other serine hydrolases as well as to a FAAH homologue, FAAH2. In addition, PF-3845-treated mice shows significant elevations in brain levels of AEA, other NAEs and liver levels of AEA, PEA and OEA. Moreover, PF-3845 is found to inhibit pain responses in a rat model of inflammatory pain. It is also found to reverse LPS-induced tactile allodynia in mice. This anti-allodynic effect requires activation of both CB1 and CB2 receptors which are the target receptors of the FAAH substrates [1, 2].
References:
[1] Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens RC, Cravatt BF. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009 Apr 24;16(4):411-20.
[2] Booker L, Kinsey SG, Abdullah RA, Blankman JL, Long JZ, Ezzili C, Boger DL, Cravatt BF, Lichtman AH. The fatty acid amide hydrolase (FAAH) inhibitor PF-3845 acts in the nervous system to reverse LPS-induced tactile allodynia in mice. Br J Pharmacol. 2012 Apr;165(8):2485-96.
- GSK2190915 sodium salt
Catalog No.:BCC5588
CAS No.:1196070-26-4
- 2-Hydroxyquinoxaline
Catalog No.:BCC8577
CAS No.:1196-57-2
- 7-Ethyl-10-Hydroxy-Camptothecin
Catalog No.:BCN8386
CAS No.:119577-28-5
- Dabrafenib Mesylate (GSK-2118436)
Catalog No.:BCC1513
CAS No.:1195768-06-9
- Dabrafenib (GSK2118436)
Catalog No.:BCC4393
CAS No.:1195765-45-7
- 11-Hydroxygelsenicine
Catalog No.:BCN4761
CAS No.:1195760-68-9
- N,N-Dimethylsphingosine
Catalog No.:BCC7959
CAS No.:119567-63-4
- Othonnine
Catalog No.:BCN2061
CAS No.:119565-25-2
- Ceanothic acid acetate
Catalog No.:BCN6083
CAS No.:119533-63-0
- Ethyllucidone
Catalog No.:BCN6082
CAS No.:1195233-59-0
- Meropenem trihydrate
Catalog No.:BCC4226
CAS No.:119478-56-7
- Fruquintinib(HMPL-013)
Catalog No.:BCC6415
CAS No.:1194506-26-7
- Olprinone Hydrochloride
Catalog No.:BCC1821
CAS No.:119615-63-3
- Sulfo-NHS-Biotin
Catalog No.:BCC3576
CAS No.:119616-38-5
- Arecaidine but-2-ynyl ester tosylate
Catalog No.:BCC6627
CAS No.:119630-77-2
- Naloxone benzoylhydrazone
Catalog No.:BCC5757
CAS No.:119630-94-3
- Moguisteine
Catalog No.:BCC4925
CAS No.:119637-67-1
- Yucalexin P-17
Catalog No.:BCN6595
CAS No.:119642-82-9
- Amadacycline methanesulfonate
Catalog No.:BCC1356
CAS No.:1196800-40-4
- 3',4'-Dihydroxyacetophenone
Catalog No.:BCN4775
CAS No.:1197-09-7
- Tranexamic acid
Catalog No.:BCN2710
CAS No.:1197-18-8
- 4-Aminophenylacetic acid
Catalog No.:BCC8687
CAS No.:1197-55-3
- 5-Methylfurmethiodide
Catalog No.:BCC6707
CAS No.:1197-60-0
- PF-05212384 (PKI-587)
Catalog No.:BCC4987
CAS No.:1197160-78-3
The fatty acid amide hydrolase inhibitor PF-3845 promotes neuronal survival, attenuates inflammation and improves functional recovery in mice with traumatic brain injury.[Pubmed:24937045]
Neuropharmacology. 2014 Oct;85:427-39.
Traumatic brain injury (TBI) is the leading cause of death in young adults in the United States, but there is still no effective agent for treatment. N-arachidonoylethanolamine (anandamide, AEA) is a major endocannabinoid in the brain. Its increase after brain injury is believed to be protective. However, the compensatory role of AEA is transient due to its rapid hydrolysis by the fatty acid amide hydrolase (FAAH). Thus, inhibition of FAAH can boost the endogenous levels of AEA and prolong its protective effect. Using a TBI mouse model, we found that post-injury chronic treatment with PF3845, a selective and potent FAAH inhibitor, reversed TBI-induced impairments in fine motor movement, hippocampus dependent working memory and anxiety-like behavior. Treatment with PF3845 inactivated FAAH activity and enhanced the AEA levels in the brain. It reduced neurodegeneration in the dentate gyrus, and up-regulated the expression of Bcl-2 and Hsp70/72 in both cortex and hippocampus. PF3845 also suppressed the increased production of amyloid precursor protein, prevented dendritic loss and restored the levels of synaptophysin in the ipsilateral dentate gyrus. Furthermore, PF3845 suppressed the expression of inducible nitric oxide synthase and cyclooxygenase-2 and enhanced the expression of arginase-1 post-TBI, suggesting a shift of microglia/macrophages from M1 to M2 phenotype. The effects of PF3845 on TBI-induced behavioral deficits and neurodegeneration were mediated by activation of cannabinoid type 1 and 2 receptors and might be attributable to the phosphorylation of ERK1/2 and AKT. These results suggest that selective inhibition of FAAH is likely to be beneficial for TBI treatment.
The fatty acid amide hydrolase (FAAH) inhibitor PF-3845 acts in the nervous system to reverse LPS-induced tactile allodynia in mice.[Pubmed:21506952]
Br J Pharmacol. 2012 Apr;165(8):2485-96.
BACKGROUND AND PURPOSE: Inflammatory pain presents a problem of clinical relevance and often elicits allodynia, a condition in which non-noxious stimuli are perceived as painful. One potential target to treat inflammatory pain is the endogenous cannabinoid (endocannabinoid) system, which is comprised of CB1 and CB2 cannabinoid receptors and several endogenous ligands, including anandamide (AEA). Blockade of the catabolic enzyme fatty acid amide hydrolase (FAAH) elevates AEA levels and elicits antinociceptive effects, without the psychomimetic side effects associated with Delta(9) -tetrahydrocannabinol (THC). EXPERIMENTAL APPROACH: Allodynia was induced by intraplantar injection of LPS. Complementary genetic and pharmacological approaches were used to determine the strategy of blocking FAAH to reverse LPS-induced allodynia. Endocannabinoid levels were quantified using mass spectroscopy analyses. KEY RESULTS: FAAH (-/-) mice or wild-type mice treated with FAAH inhibitors (URB597, OL-135 and PF-3845) displayed an anti-allodynic phenotype. Furthermore, i.p. PF-3845 increased AEA levels in the brain and spinal cord. Additionally, intraplantar PF-3845 produced a partial reduction in allodynia. However, the anti-allodynic phenotype was absent in mice expressing FAAH exclusively in the nervous system under a neural specific enolase promoter, implicating the involvement of neuronal fatty acid amides (FAAs). The anti-allodynic effects of FAAH-compromised mice required activation of both CB1 and CB2 receptors, but other potential targets of FAA substrates (i.e. micro-opioid, TRPV1 and PPARalpha receptors) had no apparent role. CONCLUSIONS AND IMPLICATIONS: AEA is the primary FAAH substrate reducing LPS-induced tactile allodynia. Blockade of neuronal FAAH reverses allodynia through the activation of both cannabinoid receptors and represents a promising target to treat inflammatory pain. LINKED ARTICLES: This article is part of a themed section on Cannabinoids in Biology and Medicine. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-8. To view Part I of Cannabinoids in Biology and Medicine visit http://dx.doi.org/10.1111/bph.2011.163.issue-7.
Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain.[Pubmed:19389627]
Chem Biol. 2009 Apr 24;16(4):411-20.
Endocannabinoids are lipid signaling molecules that regulate a wide range of mammalian behaviors, including pain, inflammation, and cognitive/emotional state. The endocannabinoid anandamide is principally degraded by the integral membrane enzyme fatty acid amide hydrolase (FAAH), and there is currently much interest in developing FAAH inhibitors to augment endocannabinoid signaling in vivo. Here, we report the discovery and detailed characterization of a highly efficacious and selective FAAH inhibitor, PF-3845. Mechanistic and structural studies confirm that PF-3845 is a covalent inhibitor that carbamylates FAAH's serine nucleophile. PF-3845 selectively inhibits FAAH in vivo, as determined by activity-based protein profiling; raises brain anandamide levels for up to 24 hr; and produces significant cannabinoid receptor-dependent reductions in inflammatory pain. These data thus designate PF-3845 as a valuable pharmacological tool for in vivo characterization of the endocannabinoid system.
Fatty acid amide hydrolase as a potential therapeutic target for the treatment of pain and CNS disorders.[Pubmed:20544003]
Expert Opin Drug Discov. 2009 Jul;4(7):763-784.
BACKGROUND: Fatty acid amide hydrolase (FAAH) is an integral membrane enzyme that hydrolyzes the endocannabinoid anandamide and related amidated signaling lipids. Genetic or pharmacological inactivation of FAAH produces analgesic, anti-inflammatory, anxiolytic, and antidepressant phenotypes without showing the undesirable side effects of direct cannabinoid receptor agonists, indicating that FAAH may be a promising therapeutic target. OBJECTIVES: This review highlights advances in the development of FAAH inhibitors of different mechanistic classes and their in vivo efficacy. Also highlighted are advances in technology for the in vitro and in vivo selectivity assessment of FAAH inhibitors employing activity-based protein profiling (ABPP) and click chemistry-ABPP, respectively. Recent reports on structure-based drug design for human FAAH generated by protein engineering using interspecies active site conversion are also discussed. METHODS: The literature searches of Medline and SciFinder databases were used. CONCLUSIONS: There has been tremendous progress in our understanding in FAAH and development of FAAH inhibitors with in vivo efficacy, selectivity, and drug like pharmacokinetic properties.


