FAAH inhibitor 1CAS# 326866-17-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 326866-17-5 | SDF | Download SDF |
| PubChem ID | 1190414 | Appearance | Powder |
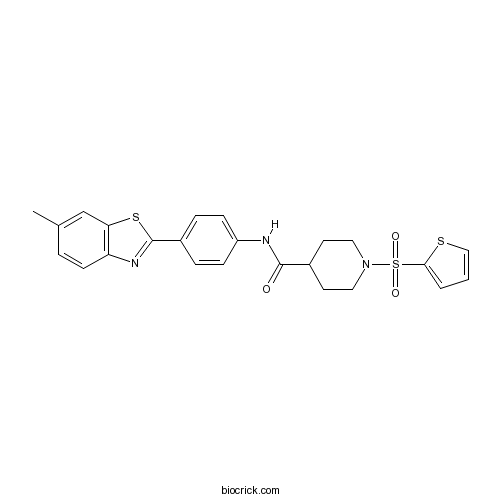
| Formula | C24H23N3O3S3 | M.Wt | 497.65 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
| Chemical Name | N-[4-(6-methyl-1,3-benzothiazol-2-yl)phenyl]-1-thiophen-2-ylsulfonylpiperidine-4-carboxamide | ||
| SMILES | CC1=CC2=C(C=C1)N=C(S2)C3=CC=C(C=C3)NC(=O)C4CCN(CC4)S(=O)(=O)C5=CC=CS5 | ||
| Standard InChIKey | IVGKSFUEBSXSAE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H23N3O3S3/c1-16-4-9-20-21(15-16)32-24(26-20)18-5-7-19(8-6-18)25-23(28)17-10-12-27(13-11-17)33(29,30)22-3-2-14-31-22/h2-9,14-15,17H,10-13H2,1H3,(H,25,28) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | FAAH inhibitor 1 is a potent fatty acid amide hydrolase (FAAH) inhibitor with an IC50 of 18±8 nM.
IC50 Value: 18±8 nM [1]
Target: FAAH
Time-dependent preincubation study of FAAH inhibitor 1 was consistent with it being a reversible inhibitor. Activity-based protein-profiling (ABPP) evaluation of FAAH inhibitors 1 in rat tissues revealed that it had exceptional selectivity and no off-target activity with respect to other serine hydrolases. Molecular shape overlay of FAAH inhibitor 1 with a known FAAH inhibitor indicated that these compounds might act as transitionstate analogues, forming putative hydrogen bonds with catalytic residues and mimicking the charge distribution of the tetrahedral transition state. FAAH inhibitors 1 was exclusively specific against FAAH in rat brain and had no missing protein bands in all the other tissues that were tested [1]. References: | |||||

FAAH inhibitor 1 Dilution Calculator

FAAH inhibitor 1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0094 mL | 10.0472 mL | 20.0944 mL | 40.1889 mL | 50.2361 mL |
| 5 mM | 0.4019 mL | 2.0094 mL | 4.0189 mL | 8.0378 mL | 10.0472 mL |
| 10 mM | 0.2009 mL | 1.0047 mL | 2.0094 mL | 4.0189 mL | 5.0236 mL |
| 50 mM | 0.0402 mL | 0.2009 mL | 0.4019 mL | 0.8038 mL | 1.0047 mL |
| 100 mM | 0.0201 mL | 0.1005 mL | 0.2009 mL | 0.4019 mL | 0.5024 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
FAAH inhibitor 1 is a potent fatty acid amide hydrolase (FAAH) inhibitor with an IC50 of 18±8 nM.
- Edpetiline
Catalog No.:BCN6771
CAS No.:32685-93-1
- H-Glu(OtBu)-OtBu.HCl
Catalog No.:BCC2934
CAS No.:32677-01-3
- Mesoridazine Besylate
Catalog No.:BCC3975
CAS No.:32672-69-8
- Fraxamoside
Catalog No.:BCN5247
CAS No.:326594-34-7
- Boc-Ser-OH
Catalog No.:BCC3439
CAS No.:3262-72-4
- Oleuropein
Catalog No.:BCN5246
CAS No.:32619-42-4
- Kaempferol 3-neohesperidoside
Catalog No.:BCN5245
CAS No.:32602-81-6
- GRI 977143
Catalog No.:BCC2401
CAS No.:325850-81-5
- BPIPP
Catalog No.:BCC7730
CAS No.:325746-94-9
- Indiplon
Catalog No.:BCC7720
CAS No.:325715-02-4
- Ascleposide E
Catalog No.:BCN5244
CAS No.:325686-49-5
- 1-Oleoyl lysophosphatidic acid sodium salt
Catalog No.:BCC7792
CAS No.:325465-93-8
- Shz 1
Catalog No.:BCC6334
CAS No.:326886-05-9
- MHY1485
Catalog No.:BCC6404
CAS No.:326914-06-1
- H-Nle-OH
Catalog No.:BCC3295
CAS No.:327-57-1
- Chlorogenic acid
Catalog No.:BCN5906
CAS No.:327-97-9
- TCS-PIM-1-4a
Catalog No.:BCC5461
CAS No.:327033-36-3
- TDZD-8
Catalog No.:BCC4258
CAS No.:327036-89-5
- VU 0285683
Catalog No.:BCC6154
CAS No.:327056-22-4
- Cyclohexanecarboxylic acid
Catalog No.:BCN3443
CAS No.:98-89-5
- tcY-NH2
Catalog No.:BCC5770
CAS No.:327177-34-4
- Heliosupine
Catalog No.:BCN1980
CAS No.:32728-78-2
- Phorbol 13-acetate
Catalog No.:BCN7231
CAS No.:32752-29-7
- Macrocarpal L
Catalog No.:BCN5248
CAS No.:327601-97-8
FAAH inhibitor, URB-597, promotes extinction and CB(1) antagonist, SR141716, inhibits extinction of conditioned aversion produced by naloxone-precipitated morphine withdrawal, but not extinction of conditioned preference produced by morphine in rats.[Pubmed:19698735]
Pharmacol Biochem Behav. 2009 Nov;94(1):154-62.
Converging evidence suggests that the endogenous cannabinoid (eCB) system is involved in extinction of learned behaviours. Using operant and classical conditioning procedures, the potential of the fatty acid amide (FAAH) inhibitor, URB-597, and the CB(1) antagonist/inverse agonist, SR141716, to promote and inhibit (respectively) extinction of learned responses previously motivated by either rewarding or aversive stimuli was investigated. In the operant conditioning procedure (Expt. 1), rats previously trained to lever press for sucrose reward were administered URB-597 (0.3 mg/kg) or the CB(1) antagonist/inverse agonist SR141716 (2.5 mg/kg) prior to each of three extinction trials. In the conditioned floor preference procedure (Expts 2a-d), rats trained to associate morphine with one of two distinctive floors were administered one of several doses of the CB(1) antagonist/inverse agonist, AM-251 (Expt 2a) or URB-597 (Expt 2b and 2d) prior to each extinction/test trial wherein a choice of both floors was presented and prior to forced exposure to each floor (Expt 2c). In the conditioned floor aversion procedure (Expt. 3), rats trained to associate a naloxone-precipitated morphine withdrawal with a floor cue were administered URB-597 or SR141716 prior to each of 24 extinction/testing trials. URB-597 did not promote and SR141716 did not reduce extinction rates for sucrose reward-induced operant responding (Expt. 1) or morphine-induced conditioned floor preference (Expts. 2a-d). In contrast, URB-597 facilitated, whereas SR141716 impaired, extinction of the conditioned floor aversion (Expt. 3). These data support previous reports that the eCB system selectively facilitates extinction of aversive memories. URB-597 may prove useful in targeting extinction of aversively motivated behaviours.
Metabolism and disposition of MM-433593, a selective FAAH-1 inhibitor, in monkeys.[Pubmed:25505606]
Pharmacol Res Perspect. 2014 Oct;2(5):e00059.
MM-433593 is a highly potent and selective inhibitor of fatty acid amide hydrolase-1 (FAAH-1) with potential utility as an orally administered treatment of pain, inflammation, and other disorders. In this study, we investigated the metabolism and pharmacokinetics of MM-433593 in monkeys, and compared plasma and urine metabolites of this compound to the in vitro metabolites produced by monkey hepatocytes. Intravenous administration of MM-433593 to cynomolgus monkeys produced a rapid distribution phase and slower elimination phase with a mean systemic clearance rate of 8-11 mL/min/kg. Absolute oral bioavailability was determined to be 14-21% with maximum plasma concentrations reached approximately 3 h (T max) following a 10 mg/kg oral dose. The average terminal half-life of MM-433593 was 17-20 h, and there were no qualitative sex differences in the metabolite profile of MM-433593. The major site of metabolism was oxidation of the methyl group at the five position of the indole ring, which was confirmed by chromatography and mass spectrometry comparison to a synthesized authentic standard. This metabolite was further oxidized to the corresponding carboxylic acid and/or conjugated with sulfate, glucuronide, or glutathione. In all, 18 metabolites were found in plasma and urine. In vitro incubations of MM-433593 with monkey hepatocytes yielded 13 metabolites, all of which were found in vivo, indicating a good correlation between the in vitro and in vivo metabolism data. A comprehensive pathway for the metabolism of MM-433593 is proposed, including a plausible, five-step biotransformation for the formation of N-acetylcysteine conjugate metabolite (M18) from the hydroxylated parent (M5).


