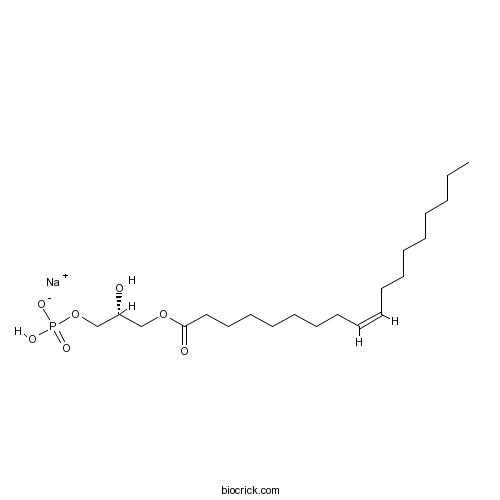
1-Oleoyl lysophosphatidic acid sodium saltActivates LPA receptor CAS# 325465-93-8 |
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- DPH
Catalog No.:BCC1538
CAS No.:484049-04-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 325465-93-8 | SDF | Download SDF |
| PubChem ID | 44159357 | Appearance | Powder |
| Formula | C21H40NaO7P | M.Wt | 458.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in phosphate buffered saline | ||
| Chemical Name | sodium;[(2R)-2-hydroxy-3-[(Z)-octadec-9-enoyl]oxypropyl] hydrogen phosphate | ||
| SMILES | CCCCCCCCC=CCCCCCCCC(=O)OCC(COP(=O)(O)[O-])O.[Na+] | ||
| Standard InChIKey | XGRLSUFHELJJAB-JGSYTFBMSA-M | ||
| Standard InChI | InChI=1S/C21H41O7P.Na/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-21(23)27-18-20(22)19-28-29(24,25)26;/h9-10,20,22H,2-8,11-19H2,1H3,(H2,24,25,26);/q;+1/p-1/b10-9-;/t20-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous agonist of the lysophospholipid receptors LPA1 and LPA2. Inhibits differentiation of neural stem cells (NSCs) into neurons. |

1-Oleoyl lysophosphatidic acid sodium salt Dilution Calculator

1-Oleoyl lysophosphatidic acid sodium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.181 mL | 10.9051 mL | 21.8103 mL | 43.6205 mL | 54.5256 mL |
| 5 mM | 0.4362 mL | 2.181 mL | 4.3621 mL | 8.7241 mL | 10.9051 mL |
| 10 mM | 0.2181 mL | 1.0905 mL | 2.181 mL | 4.3621 mL | 5.4526 mL |
| 50 mM | 0.0436 mL | 0.2181 mL | 0.4362 mL | 0.8724 mL | 1.0905 mL |
| 100 mM | 0.0218 mL | 0.1091 mL | 0.2181 mL | 0.4362 mL | 0.5453 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: N/A
1-Oleoyl lysophosphatidic acid (LPA) is a naturally occurring phospholipid with various effects. LPA is widely distributed in many tissues. Similar to many other biomediators, LPA interacts with cells via specific cell surface receptors, such as G proteincoupled receptors, to induce biological effects.
In vitro: LPA could mediate signal transduction via Edg/LPA receptor, inducing the proliferation, migration, adhesion and antiapoptotic function of tumor cells. Additionally, LPA was found to elicit upregulation of VEGF, leading to an indirect influence on initiation and progression of malignancies. LPA could also stimulate matrix metalloproteinase secretion and tumor angiogenesis factor [1].
In vivo: LPA was tested for its vascular remodeling effect in a rat model of hypoxic pulmonary hypertension. Serum from animals in the hypoxic group showed higher chemoattractant properties toward rat primary lung fibroblasts, and such cell migration increase was prevented by the LPA receptor 1 and 3 antagonists. LPA also found to increase adhesive properties of human pulmonary artery endothelial cells, via the activation of LPA receptor 1 or 3 [1].
Clinical trial: Clinical retults showed that LPA concentration was significantly higher in pancreatic cancer patients. For diagnosis of pancreatic cancer, the sensitivity of LPA was 89.6% and the specificity 79. 4%. However, plasma LPA alteration had shown significant correlations with the tumor size, pathological stage, surrounding lymph nodes, as well as specific histopathological features [2].
References:
[1] Yongling G, Shaokai W, Chenjie T, Jinfei C, Shukui W, Xiufeng C, Guangmei L, Pin L. Clinical evaluation on the determination of plasma lysophosphatidic acid concentration in Chinese human pancreatic cancer. International Journal of Hepatobiliary and Pancreatic Diseases 2011;1:6-12.
[2] Shlyonsky V,Naeije R,Mies F. Possible role of lysophosphatidic acid in rat model of hypoxic pulmonary vascular remodeling. Pulm Circ.2014 Sep;4(3):471-81.
- ICA 110381
Catalog No.:BCC6338
CAS No.:325457-99-6
- Sulfo-NHS-SS-Biotin
Catalog No.:BCC3580
CAS No.:325143-98-4
- Ergosta-7,22-dien-3-one
Catalog No.:BCN7088
CAS No.:32507-77-0
- Isorhapotogenin
Catalog No.:BCN3383
CAS No.:32507-66-7
- Myricanone
Catalog No.:BCN5243
CAS No.:32492-74-3
- Periplocymarin
Catalog No.:BCN8485
CAS No.:32476-67-8
- T 0156 hydrochloride
Catalog No.:BCC5803
CAS No.:324572-93-2
- 1-Indanamine hydrochloride
Catalog No.:BCC8467
CAS No.:32457-23-1
- NPS-2143 hydrochloride
Catalog No.:BCC1808
CAS No.:324523-20-8
- Isochlorogenic acid
Catalog No.:BCN5910
CAS No.:534-61-2
- 20(29)-Lupene-3,23-diol
Catalog No.:BCN5242
CAS No.:32451-85-7
- Khasianine
Catalog No.:BCN2530
CAS No.:32449-98-2
- Ascleposide E
Catalog No.:BCN5244
CAS No.:325686-49-5
- Indiplon
Catalog No.:BCC7720
CAS No.:325715-02-4
- BPIPP
Catalog No.:BCC7730
CAS No.:325746-94-9
- GRI 977143
Catalog No.:BCC2401
CAS No.:325850-81-5
- Kaempferol 3-neohesperidoside
Catalog No.:BCN5245
CAS No.:32602-81-6
- Oleuropein
Catalog No.:BCN5246
CAS No.:32619-42-4
- Boc-Ser-OH
Catalog No.:BCC3439
CAS No.:3262-72-4
- Fraxamoside
Catalog No.:BCN5247
CAS No.:326594-34-7
- Mesoridazine Besylate
Catalog No.:BCC3975
CAS No.:32672-69-8
- H-Glu(OtBu)-OtBu.HCl
Catalog No.:BCC2934
CAS No.:32677-01-3
- Edpetiline
Catalog No.:BCN6771
CAS No.:32685-93-1
- FAAH inhibitor 1
Catalog No.:BCC4254
CAS No.:326866-17-5
Lysophosphatidic acid signals through multiple receptors in osteoclasts to elevate cytosolic calcium concentration, evoke retraction, and promote cell survival.[Pubmed:20551326]
J Biol Chem. 2010 Aug 13;285(33):25792-801.
Lysophosphatidic acid (LPA) is a bioactive phospholipid whose functions are mediated by multiple G protein-coupled receptors. We have shown that osteoblasts produce LPA, raising the possibility that it mediates intercellular signaling among osteoblasts and osteoclasts. Here we investigated the expression, signaling and function of LPA receptors in osteoclasts. Focal application of LPA elicited transient increases in cytosolic calcium concentration ([Ca(2+)](i)), with 50% of osteoclasts responding at approximately 400 nm LPA. LPA-induced elevation of [Ca(2+)](i) was blocked by pertussis toxin or the LPA(1/3) receptor antagonist VPC-32183. LPA caused sustained retraction of osteoclast lamellipodia and disrupted peripheral actin belts. Retraction was insensitive to VPC-32183 or pertussis toxin, indicating involvement of a distinct signaling pathway. In this regard, inhibition of Rho-associated kinase stimulated respreading after LPA-induced retraction. Real-time reverse transcription-PCR revealed transcripts encoding LPA(1) and to a lesser extent LPA(2), LPA(4), and LPA(5) receptor subtypes. LPA induced nuclear translocation of NFATc1 and enhanced osteoclast survival, effects that were blocked by VPC-32183 or by a specific peptide inhibitor of NFAT activation. LPA slightly reduced the resorptive activity of osteoclasts in vitro. Thus, LPA binds to at least two receptor subtypes on osteoclasts: LPA(1), which couples through G(i/o) to elevate [Ca(2+)](i), activate NFATc1, and promote survival, and a second receptor that likely couples through G(12/13) and Rho to evoke and maintain retraction through reorganization of the actin cytoskeleton. These findings reveal a signaling axis in bone through which LPA, produced by osteoblasts, acts on multiple receptor subtypes to induce pleiotropic effects on osteoclast activity and function.
Lysophosphatidic acid inhibits neuronal differentiation of neural stem/progenitor cells derived from human embryonic stem cells.[Pubmed:18308941]
Stem Cells. 2008 May;26(5):1146-54.
Lysophospholipids are signaling molecules that play broad and major roles within the nervous system during both early development and neural injury. We used neural differentiation of human embryonic stem cells (hESC) as an in vitro model to examine the specific effects of lysophosphatidic acid (LPA) at various stages of neural development, from neural induction to mature neurons and glia. We report that LPA inhibits neurosphere formation and the differentiation of neural stem cells (NSC) toward neurons, without modifying NSC proliferation, apoptosis, or astrocytic differentiation. LPA acts through the activation of the Rho/ROCK and the phosphatidylinositol 3-kinase/Akt pathways to inhibit neuronal differentiation. This study is the first demonstration of a role for LPA signaling in neuronal differentiation of hESC. As LPA concentrations increase during inflammation, the inhibition of neuronal differentiation by LPA might contribute to the low level of neurogenesis observed following neurotrauma.
Mitogenic action of lysophosphatidic acid and phosphatidic acid on fibroblasts. Dependence on acyl-chain length and inhibition by suramin.[Pubmed:1731751]
Biochem J. 1992 Jan 1;281 ( Pt 1):163-9.
Lysophosphatidic acid (LPA) is a naturally occurring phospholipid with growth-factor-like activities [van Corven, Groenink, Jalink, Eichholtz & Moolenaar (1989) Cell 45, 45-54]. We have examined various structural analogues of LPA for their ability to stimulate DNA synthesis in quiescent fibroblasts. When the acyl-chain length is varied, the rank order of mitogenic potency is: 1-oleoyl LPA congruent to 1-palmitoyl LPA greater than 1-myristoyl LPA greater than 1-lauroyl LPA greater than 1-decanoyl LPA; the last compound shows almost no activity over the concentration range tested (1-100 microM). An ether-linked LPA (1-O-hexadecylglycerol 3-phosphate) has much decreased mitogenic activity as compared with the ester-linked analogue at concentrations less than 25 microM, and becomes cytotoxic at higher concentrations. Hexadecylphosphate, which lacks a glycerol backbone, has negligible activity. On a molar basis, diacyl phosphatidic acid (PA) is about equally potent as the corresponding LPA analogue, showing similar acyl-chain-length dependence; the data argue against the possibility that the mitogenic action of PA is due to contaminating traces of LPA. Although the short-chain analogues of LPA and PA fail to antagonize the action of long-chain (L)PAs, the polyanionic drug suramin inhibits LPA- and PA-induced, DNA synthesis in a reversible and dose-dependent manner, at concentrations [IC50 (concn. giving 50% inhibition) approximately 70 microM] that do not affect epidermal-growth-factor-induced DNA synthesis. Suramin appears to act in the early G0/G1 phase of the cell cycle, blocking immediate responses to LPA such as phosphoinositide hydrolysis. We conclude that both LPA and PA can function as growth-promoting phospholipids, with the fatty acid chain length being a major determinant of mitogenic potency.


