PeriplocymarinCAS# 32476-67-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 32476-67-8 | SDF | Download SDF |
| PubChem ID | 12305974 | Appearance | White crystalline powder |
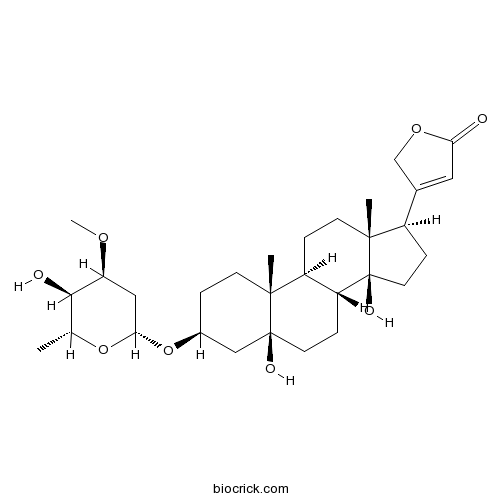
| Formula | C30H46O8 | M.Wt | 534.68 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-[(3S,5S,8R,9S,10R,13R,14S,17R)-5,14-dihydroxy-3-[(2R,4S,5R,6R)-5-hydroxy-4-methoxy-6-methyloxan-2-yl]oxy-10,13-dimethyl-2,3,4,6,7,8,9,11,12,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]-2H-furan-5-one | ||
| SMILES | CC1C(C(CC(O1)OC2CCC3(C4CCC5(C(CCC5(C4CCC3(C2)O)O)C6=CC(=O)OC6)C)C)OC)O | ||
| Standard InChIKey | XRWQBDJPMXRDOQ-YUUDFPFBSA-N | ||
| Standard InChI | InChI=1S/C30H46O8/c1-17-26(32)23(35-4)14-25(37-17)38-19-5-9-27(2)21-6-10-28(3)20(18-13-24(31)36-16-18)8-12-30(28,34)22(21)7-11-29(27,33)15-19/h13,17,19-23,25-26,32-34H,5-12,14-16H2,1-4H3/t17-,19+,20-,21+,22-,23+,25+,26-,27-,28-,29+,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Periplocymarin, a cardiac glycoside, has potential anti-cancer activity. 2. Octreotide-conjugated periplocymarin demonstrates tumor selectivity and may be useful as a targeting agent to improve the safety profile of cardiac glycosides for cancer therapy. |
| Targets | Calcium Channel | Sodium Channel | ATPase | Potassium Channel | P-gp | P450 (e.g. CYP17) |

Periplocymarin Dilution Calculator

Periplocymarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8703 mL | 9.3514 mL | 18.7028 mL | 37.4056 mL | 46.7569 mL |
| 5 mM | 0.3741 mL | 1.8703 mL | 3.7406 mL | 7.4811 mL | 9.3514 mL |
| 10 mM | 0.187 mL | 0.9351 mL | 1.8703 mL | 3.7406 mL | 4.6757 mL |
| 50 mM | 0.0374 mL | 0.187 mL | 0.3741 mL | 0.7481 mL | 0.9351 mL |
| 100 mM | 0.0187 mL | 0.0935 mL | 0.187 mL | 0.3741 mL | 0.4676 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- T 0156 hydrochloride
Catalog No.:BCC5803
CAS No.:324572-93-2
- 1-Indanamine hydrochloride
Catalog No.:BCC8467
CAS No.:32457-23-1
- NPS-2143 hydrochloride
Catalog No.:BCC1808
CAS No.:324523-20-8
- Isochlorogenic acid
Catalog No.:BCN5910
CAS No.:534-61-2
- 20(29)-Lupene-3,23-diol
Catalog No.:BCN5242
CAS No.:32451-85-7
- Khasianine
Catalog No.:BCN2530
CAS No.:32449-98-2
- H-D-Cys-OH.H2O.HCl
Catalog No.:BCC2913
CAS No.:32443-99-5
- Isofebrifugine
Catalog No.:BCN3270
CAS No.:32434-44-9
- Vitexin-4'-Rhamnoside(Rg)
Catalog No.:BCC8369
CAS No.:32426-34-9
- AKTide-2T
Catalog No.:BCC5908
CAS No.:324029-01-8
- Dexfenfluramine hydrochloride
Catalog No.:BCC5927
CAS No.:3239-45-0
- Imetit dihydrobromide
Catalog No.:BCC6768
CAS No.:32385-58-3
- Myricanone
Catalog No.:BCN5243
CAS No.:32492-74-3
- Isorhapotogenin
Catalog No.:BCN3383
CAS No.:32507-66-7
- Ergosta-7,22-dien-3-one
Catalog No.:BCN7088
CAS No.:32507-77-0
- Sulfo-NHS-SS-Biotin
Catalog No.:BCC3580
CAS No.:325143-98-4
- ICA 110381
Catalog No.:BCC6338
CAS No.:325457-99-6
- 1-Oleoyl lysophosphatidic acid sodium salt
Catalog No.:BCC7792
CAS No.:325465-93-8
- Ascleposide E
Catalog No.:BCN5244
CAS No.:325686-49-5
- Indiplon
Catalog No.:BCC7720
CAS No.:325715-02-4
- BPIPP
Catalog No.:BCC7730
CAS No.:325746-94-9
- GRI 977143
Catalog No.:BCC2401
CAS No.:325850-81-5
- Kaempferol 3-neohesperidoside
Catalog No.:BCN5245
CAS No.:32602-81-6
- Oleuropein
Catalog No.:BCN5246
CAS No.:32619-42-4
A validated LC-MS/MS assay for the simultaneous determination of periplocin and its two metabolites, periplocymarin and periplogenin in rat plasma: Application to a pharmacokinetic study.[Pubmed:26093244]
J Pharm Biomed Anal. 2015 Oct 10;114:292-5.
A sensitive and reliable LC-MS/MS method was developed and validated for the simultaneous determination of periplocin and its two metabolites (Periplocymarin and periplogenin) in rat plasma using psoralen as the internal standard (IS). After liquid-liquid extraction with ethyl acetate, chromatographic separation was performed on a C18 column with a 13 min gradient elution using 0.1% formic acid and acetonitrile as mobile phase at a flow rate of 0.3 mL/min. The detection was accomplished on a tandem mass spectrometer via an electrospray ionization (ESI) source by multiple reaction monitoring (MRM) in the positive ionization mode. The lower limits of quantitation (LLOQs) for periplocin, Periplocymarin and periplogenin were 0.5, 1 and 0.1 ng/mL, respectively. The mean recoveries of the analytes and IS were higher than 67.7%. The proposed method was successfully applied to evaluating the pharmacokinetic studies of periplocin and its metabolites (Periplocymarin and periplogenin) in rats after a single oral administration of periplocin at 50 mg/kg.
Quantitative determination of periplocymarin in rat plasma and tissue by LC-MS/MS: application to pharmacokinetic and tissue distribution study.[Pubmed:26663385]
Biomed Chromatogr. 2016 Aug;30(8):1195-201.
A simple, rapid and sensitive liquid chromatography with tandem mass spectrometry (LC-MS/MS) method for the determination of Periplocymarin in biological samples was developed and successfully applied to the pharmacokinetic and tissue distribution study of Periplocymarin after oral administration of periplocin. Biological samples were processed with ethyl acetate by liquid-liquid extraction, and diazepam was used as the internal standard. Periplocymarin was analyzed on a C18 column with isocratic eluted mobile phase composed of methanol and water (containing 0.1% formic acid) at a flow rate of 0.2 mL/min (73:27, v/v). Detection was performed on a triple-quadrupole tandem mass spectrometer using positive-ion mode electrospray ionization in the selected reaction monitoring mode. The MS/MS ion transitions monitored were m/z 535.3-->355.1 and 285.1-->193.0 for Periplocymarin and diazepam, respectively. Good linearity was observed over the concentration ranges. The lower limit of quantification was 0.5 ng/mL in plasma and tested tissues. The intra-and inter-day precisions (relative standard deviation) were <10.2 and 10.5%, respectively, and accuracies (relative error) were between -6.8 and 8.9%. Recoveries in plasma and tissue were >90%. The validated method was successfully applied to the pharmacokinetic and tissue distribution studies of Periplocymarin in rats. Copyright (c) 2016 John Wiley & Sons, Ltd.
Periplocymarin is a potential natural compound for drug development: highly permeable with absence of P-glycoprotein efflux and cytochrome P450 inhibitions.[Pubmed:24424534]
Biopharm Drug Dispos. 2014 May;35(4):195-206.
Periplocymarin, a cardiac glycoside isolated from Periploca sepium (P. sepium) and Periploca graeca (P. graeca), is a potential anti-cancer compound. The aim of the study was to investigate the potential for Periplocymarin to interact with P-glycoprotein (P-gp) and to inhibit cytochrome P450s known to be expressed in the human small intestine. The in vitro and in situ permeability of Periplocymarin were studied using Madin-Darby canine kidney (MDCK-II-WT) cells transfected with or without the human multidrug resistance (MDR1) gene and the single-pass perfused rat intestinal model. The cell system exhibited high functional activity and a net efflux ratio (NER) of 4.32 after transport of Rhodamine 123 (R123) (the P-gp substrate). Periplocymarin is highly permeable (Papp > 10 x 10(-6) cm/s; Peff(rat) > 5.09 x 10(-5) cm/s) and independent of P-gp influences. The NER at 100 mum Periplocymarin (0.8) was unchanged in the presence of cyclosporine A (a non-specific P-gp inhibitor) (0.82). In the single-pass intestinal model, the Peff (rat) of 5 microg/ml Periplocymarin (5.490 x 10(-5) cm/s) did not change in the presence of cyclosporine A (5.394 x 10(-5) cm/s). In the R123-inhibition assay, Periplocymarin did not competitively inhibit P-gp. The inhibitory potential of Periplocymarin on cytochrome P450 (CYP450s) was also studied. Periplocymarin (5, 50 mum) did not inhibit CYP1A2, CYP2C9, CYP2C19, CYP2D6 or CYP3A4. Thus, Periplocymarin is unlikely to encounter drug-drug interactions with P-gp and CYP450s. Periplocymarin could be taken forward for further studies in drug development to test bioavailability, Phase II enzyme interactions and additional transporter interactions.
Octreotide-periplocymarin conjugate prodrug for improving targetability and anti-tumor efficiency: synthesis, in vitro and in vivo evaluation.[Pubmed:27861145]
Oncotarget. 2016 Dec 27;7(52):86326-86338.
Cardiac glycosides could increase intracellular Ca2+ ion by inhibiting the Na+/K+ATPase to induce apoptosis in many tumor cells. However, narrow therapeutic index, poor tumor selectivity and severe cardiovascular toxicity hinder their applications in cancer treatment. To improve the safety profile and tumor targetablility of cardiac glycosides, we designed octreotide conjugated Periplocymarin, a cardiac glycoside isolated from Cortex periplocae. The conjugate showed higher cytotoxicity on MCF-7 cells and HepG2 tumor cells (SSTRs overexpression) but much less toxicity in L-02 normal cells. Tissue distribution studies of the conjugate using H22 tumor model in mice showed higher accumulation in tumor and lower distribution in heart and liver than Periplocymarin. Furthermore, in vivo anticancer effects of the conjugate on mice bearing H22 cancer xenografts confirmed enhanced anti-tumor efficacy and decreased systemic toxicity. Altogether, octreotide-conjugated Periplocymarin demonstrated tumor selectivity and may be useful as a targeting agent to improve the safety profile of cardiac glycosides for cancer therapy.


