DPHc-ABL activitor CAS# 484049-04-9 |
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Nilotinib(AMN-107)
Catalog No.:BCC3643
CAS No.:641571-10-0
- Ponatinib (AP24534)
Catalog No.:BCC2522
CAS No.:943319-70-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 484049-04-9 | SDF | Download SDF |
| PubChem ID | 660311 | Appearance | Powder |
| Formula | C18H13FN4O2 | M.Wt | 336.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (297.34 mM; Need ultrasonic) | ||
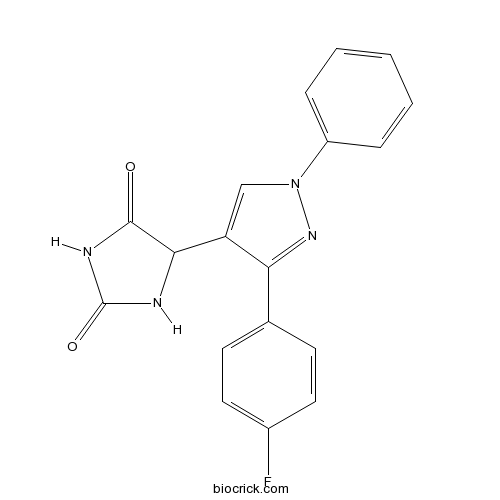
| Chemical Name | 5-[3-(4-fluorophenyl)-1-phenylpyrazol-4-yl]imidazolidine-2,4-dione | ||
| SMILES | C1=CC=C(C=C1)N2C=C(C(=N2)C3=CC=C(C=C3)F)C4C(=O)NC(=O)N4 | ||
| Standard InChIKey | MPQWYPLPWGUMJE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H13FN4O2/c19-12-8-6-11(7-9-12)15-14(16-17(24)21-18(25)20-16)10-23(22-15)13-4-2-1-3-5-13/h1-10,16H,(H2,20,21,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | DPH is a potent cell permeable c-Abl activator, which displays potent enzymatic and cellular activity in stimulating c-Abl activation.In Vitro:DPH binds to the myristoyl binding site and prevents the formation of the bent conformation of the αI helix through steric hindrance, a mode of action distinct from the previously identified allosteric c-Abl inhibitor, GNF-2, that also binds to the myristoyl binding site. DPH represents the first cell-permeable, small-molecule tool compound for c-Abl activation[1]. References: | |||||

DPH Dilution Calculator

DPH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9733 mL | 14.8664 mL | 29.7327 mL | 59.4654 mL | 74.3318 mL |
| 5 mM | 0.5947 mL | 2.9733 mL | 5.9465 mL | 11.8931 mL | 14.8664 mL |
| 10 mM | 0.2973 mL | 1.4866 mL | 2.9733 mL | 5.9465 mL | 7.4332 mL |
| 50 mM | 0.0595 mL | 0.2973 mL | 0.5947 mL | 1.1893 mL | 1.4866 mL |
| 100 mM | 0.0297 mL | 0.1487 mL | 0.2973 mL | 0.5947 mL | 0.7433 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
DPH is c-ABL activitor.
- Okanin
Catalog No.:BCN6475
CAS No.:484-76-4
- Isodictamnine
Catalog No.:BCN7066
CAS No.:484-74-2
- Angiotensin I (human, mouse, rat)
Catalog No.:BCC1004
CAS No.:484-42-4
- Dictamnine
Catalog No.:BCN1273
CAS No.:484-29-7
- Bergapten
Catalog No.:BCN5582
CAS No.:484-20-8
- 9-Phenanthrol
Catalog No.:BCC7989
CAS No.:484-17-3
- Osthenol
Catalog No.:BCN8342
CAS No.:484-14-0
- Osthol
Catalog No.:BCN5581
CAS No.:484-12-8
- Chrysophanol 1-glucoside
Catalog No.:BCC8146
CAS No.:4839-60-5
- N-Demethylloine
Catalog No.:BCN2004
CAS No.:4839-19-4
- N4-Benzoyl-2'-deoxycytidine
Catalog No.:BCC9071
CAS No.:4836-13-9
- Purmorphamine
Catalog No.:BCC3641
CAS No.:483367-10-8
- Brucine sulfate
Catalog No.:BCN2460
CAS No.:4845-99-2
- ProTx I
Catalog No.:BCC6255
CAS No.:484598-35-8
- ProTx II
Catalog No.:BCC6103
CAS No.:484598-36-9
- N-Nornuciferine
Catalog No.:BCN4048
CAS No.:4846-19-9
- Aristolochic acid C
Catalog No.:BCN2658
CAS No.:4849-90-5
- Reticuline
Catalog No.:BCN5583
CAS No.:485-19-8
- Cytisine
Catalog No.:BCN6270
CAS No.:485-35-8
- (+)-Bicuculline
Catalog No.:BCN1238
CAS No.:485-49-4
- Cinchonidine
Catalog No.:BCC5316
CAS No.:485-71-2
- Formononetin
Catalog No.:BCN1061
CAS No.:485-72-3
- Hydrangetin
Catalog No.:BCN7439
CAS No.:485-90-5
- 5,7,3'-Trihydroxyflavanone
Catalog No.:BCC8269
CAS No.:104732-07-2
Quantitative Monitoring of Microphase Separation Behaviors in Cationic Liposomes Using HHC, DPH, and Laurdan: Estimation of the Local Electrostatic Potentials in Microdomains.[Pubmed:27022833]
Langmuir. 2016 Apr 19;32(15):3630-6.
Microphase separation behaviors of cationic liposomes have been investigated using a pH-sensitive fluorescent probe with 4-heptadecyl-7-hydroxycoumarin (HHC), 1,6-diphenyl-1,3,5-hexatriene, and 6-lauroyl-2-dimethylaminonaphthalene, and to estimate localized electrostatic potentials. Shifts of the apparent pKa values of HHC were observed in cationic liposomes in proportion to the amount of cationic lipids. Two pKa values were obtained with 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC)/3beta-[N(N',N'-dimethylaminoethane)-carbamoyl] cholesterol hydrochloride (DC-Ch) liposomes, while only one pKa value was generated with either DOPC/1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) or DOPC/dimethyldioctadecylammonium-bromide (DODAB) liposomes. The physicochemical membrane property analyses, focusing on membrane fluidity and membrane polarity, revealed heterogeneity among DOPC/DC-Ch liposomes. By analyzing the pH titration curves using sigmoidal fitting, the localized electrostatic potentials were estimated. For DOPC/DOTAP = (7/3), the membrane was in the liquid-disordered phase and the density of cationic molecules was 0.41 cation/nm(2). For DOPC/DC-Ch = (7/3), the membrane was heterogeneous and the densities of cationic molecules in liquid-disordered and liquid-ordered phases were 0.25 and 1.24 cation/nm(2), respectively. We thereby conclude that the DC-Ch molecules can form nanodomains when these molecules are concentrated to 59%.
The pharmacokinetics of DPH after the administration of a single intravenous or intramuscular dose in healthy dogs.[Pubmed:26813802]
J Vet Pharmacol Ther. 2016 Oct;39(5):452-9.
The objective of this study was to determine the pharmacokinetics of diphenhydramine (DPH) in healthy dogs following a single i.v. or i.m. dose. Dogs were randomly allocated in two treatment groups and received DPH at 1 mg/kg, i.v., or 2 mg/kg, i.m. Blood samples were collected serially over 24 h. Plasma concentrations of DPH were determined by high-performance liquid chromatography, and noncompartmental pharmacokinetic analysis was performed with the commercially available software. Cardio-respiratory parameters, rectal temperature and effects on behaviour, such as sedation or excitement, were recorded. Diphenhydramine Clarea , Vdarea and T1/2 were 20.7 +/- 2.9 mL/kg/min, 7.6 +/- 0.7 L/kg and 4.2 +/- 0.5 h for the i.v. route, respectively, and Clarea /F, Vdarea /F and T1/2 20.8 +/- 2.7 mL/kg/min, 12.3 +/- 1.2 L/kg and 6.8 +/- 0.7 h for the i.m. route, respectively. Bioavailability was 88% after i.m. administration. No significant differences were found in physiological parameters between groups or within dogs of the same group, and values remained within normal limits. No adverse effects or changes in mental status were observed after the administration of DPH. Both routes of administration resulted in DPH plasma concentrations which exceeded levels considered therapeutic in humans.
Diphenylhexatriene membrane probes DPH and TMA-DPH: A comparative molecular dynamics simulation study.[Pubmed:27475296]
Biochim Biophys Acta. 2016 Nov;1858(11):2647-2661.
Fluorescence spectroscopy and microscopy have been utilized as tools in membrane biophysics for decades now. Because phospholipids are non-fluorescent, the use of extrinsic membrane probes in this context is commonplace. Among the latter, 1,6-diphenylhexatriene (DPH) and its trimethylammonium derivative (TMA-DPH) have been extensively used. It is widely believed that, owing to its additional charged group, TMA-DPH is anchored at the lipid/water interface and reports on a bilayer region that is distinct from that of the hydrophobic DPH. In this study, we employ atomistic MD simulations to characterize the behavior of DPH and TMA-DPH in 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and POPC/cholesterol (4:1) bilayers. We show that although the dynamics of TMA-DPH in these membranes is noticeably more hindered than that of DPH, the location of the average fluorophore of TMA-DPH is only ~3-4A more shallow than that of DPH. The hindrance observed in the translational and rotational motions of TMA-DPH compared to DPH is mainly not due to significant differences in depth, but to the favorable electrostatic interactions of the former with electronegative lipid atoms instead. By revealing detailed insights on the behavior of these two probes, our results are useful both in the interpretation of past work and in the planning of future experiments using them as membrane reporters.


