Angiotensin I (human, mouse, rat)Precursor of angiotensin II CAS# 484-42-4 |
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- BMS-345541
Catalog No.:BCC1423
CAS No.:547757-23-3
- Bay 65-1942 free base
Catalog No.:BCC1408
CAS No.:600734-02-9
- Bay 65-1942 HCl salt
Catalog No.:BCC1409
CAS No.:600734-06-3
- Bay 65-1942 R form
Catalog No.:BCC1410
CAS No.:758683-21-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 484-42-4 | SDF | Download SDF |
| PubChem ID | 3081372 | Appearance | Powder |
| Formula | C62H89N17O14 | M.Wt | 1296.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 25 mg/mL (19.28 mM; Need ultrasonic) | ||
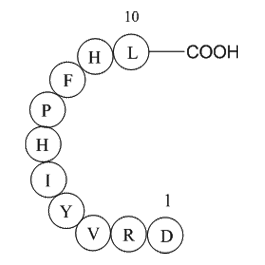
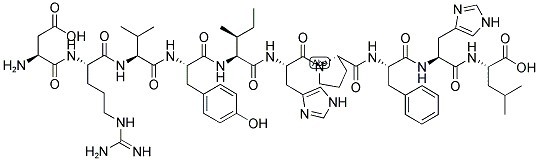
| Sequence | DRVYIHPFHL | ||
| Chemical Name | (2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-1-[(2S)-2-[[(2S,3S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-3-carboxypropanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-methylpentanoyl]amino]-3-(1H-imidazol-5-yl)propanoyl]pyrrolidine-2-carbonyl]amino]-3-phenylpropanoyl]amino]-3-(1H-imidazol-5-yl)propanoyl]amino]-4-methylpentanoic acid | ||
| SMILES | CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc2[nH]cnc2)C(=O)N3CCC[C@H]3C(=O)N[C@@H](Cc4ccccc4)C(=O)N[C@@H](Cc5[nH]cnc5)C(=O)N[C@@H](CC(C)C)C(O)=O | ||
| Standard InChIKey | ORWYRWWVDCYOMK-HBZPZAIKSA-N | ||
| Standard InChI | InChI=1S/C62H89N17O14/c1-7-35(6)51(78-56(87)44(25-37-17-19-40(80)20-18-37)74-58(89)50(34(4)5)77-53(84)42(15-11-21-68-62(64)65)71-52(83)41(63)28-49(81)82)59(90)75-46(27-39-30-67-32-70-39)60(91)79-22-12-16-48(79)57(88)73-43(24-36-13-9-8-10-14-36)54(85)72-45(26-38-29-66-31-69-38)55(86)76-47(61(92)93)23-33(2)3/h8-10,13-14,17-20,29-35,41-48,50-51,80H,7,11-12,15-16,21-28,63H2,1-6H3,(H,66,69)(H,67,70)(H,71,83)(H,72,85)(H,73,88)(H,74,89)(H,75,90)(H,76,86)(H,77,84)(H,78,87)(H,81,82)(H,92,93)(H4,64,65,68)/t35-,41-,42-,43-,44-,45-,46-,47-,48-,50-,51-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous peptide substrate for angiotensin converting enzyme (ACE); precursor to the vasoconstrictor peptide angiotensin II. |

Angiotensin I (human, mouse, rat) Dilution Calculator

Angiotensin I (human, mouse, rat) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Angiotensin I (Ang I) (C62H89N17O14), with the sequence H-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu-OH, is formed by the action of renin on angiotensinogen. Renin is produced in the kidneys in response to renal sympathetic activity, decreased intrarenal blood pressure (<90mmHg systolic blood pressure) at the juxtaglomerular cells. Ang I appears to have no biological activity and exists solely as a precursor to angiotensin II (A II). Ang I is cleaved to Ang II by the angiotensin-converting enzyme (ACE). Ang II increases blood pressure by stimulating the Gq protein in vascular smooth muscle cells (which in turn activates contraction by an IP3-dependent mechanism).
Ref:
1. Lundequist, A. et al. J. Biol. Chem. 279, 32339 (2004); Olson, S. et al. Am. J. Physiol. Lung. Cell Mol. Physiol. 287, L559 (2004); Sanker, S. et al. J. Biol. Chem. 272, 2963 (1997).
2. Preston RA, Materson BJ, Reda DJ, et al. Age-Race Subgroup Compared With Renin Profile as Predictors of Blood Pressure Response to Antihypertensive Therapy. JAMA. 1998;280(13):1168-1172. doi:10.1001/jama.280.13.1168.
- Dictamnine
Catalog No.:BCN1273
CAS No.:484-29-7
- Bergapten
Catalog No.:BCN5582
CAS No.:484-20-8
- 9-Phenanthrol
Catalog No.:BCC7989
CAS No.:484-17-3
- Osthenol
Catalog No.:BCN8342
CAS No.:484-14-0
- Osthol
Catalog No.:BCN5581
CAS No.:484-12-8
- Chrysophanol 1-glucoside
Catalog No.:BCC8146
CAS No.:4839-60-5
- N-Demethylloine
Catalog No.:BCN2004
CAS No.:4839-19-4
- N4-Benzoyl-2'-deoxycytidine
Catalog No.:BCC9071
CAS No.:4836-13-9
- Purmorphamine
Catalog No.:BCC3641
CAS No.:483367-10-8
- SB-674042
Catalog No.:BCC1931
CAS No.:483313-22-0
- Luvangetin
Catalog No.:BCN7527
CAS No.:483-92-1
- Calycanthoside
Catalog No.:BCN5580
CAS No.:483-91-0
- Isodictamnine
Catalog No.:BCN7066
CAS No.:484-74-2
- Okanin
Catalog No.:BCN6475
CAS No.:484-76-4
- DPH
Catalog No.:BCC1538
CAS No.:484049-04-9
- Brucine sulfate
Catalog No.:BCN2460
CAS No.:4845-99-2
- ProTx I
Catalog No.:BCC6255
CAS No.:484598-35-8
- ProTx II
Catalog No.:BCC6103
CAS No.:484598-36-9
- N-Nornuciferine
Catalog No.:BCN4048
CAS No.:4846-19-9
- Aristolochic acid C
Catalog No.:BCN2658
CAS No.:4849-90-5
- Reticuline
Catalog No.:BCN5583
CAS No.:485-19-8
- Cytisine
Catalog No.:BCN6270
CAS No.:485-35-8
- (+)-Bicuculline
Catalog No.:BCN1238
CAS No.:485-49-4
- Cinchonidine
Catalog No.:BCC5316
CAS No.:485-71-2
Potency and selectivity of RXP407 on human, rat, and mouse angiotensin-converting enzyme.[Pubmed:11274969]
Biochem Pharmacol. 2001 Apr 1;61(7):835-41.
By screening phosphinic peptide libraries, we recently reported the discovery of RXP407 (Ac-Asp-PheY(PO2-CH2)LAla-Ala-NH2), a potent N-domain-selective inhibitor of recombinant human angiotensin-converting enzyme (ACE). Preliminary studies to evaluate the in vivo activity of RXP407 in rat led us to suspect possible differences in the binding property of RXP407 between human and rat ACE. The aim of the present study was thus to determine the potency of RXP407 toward rat and mouse ACEs, as compared to non-recombinant human ACE, and to assess the efficacy of this inhibitor in discriminating between the N- and C-domains of these ACE enzymes. By comparing the ability of RXP407 to block purified somatic and germinal ACE from mice, RXP407 was shown to be a potent N-domain-selective inhibitor of mouse somatic ACE, a behavior similar to that observed with human somatic ACE. In contrast, RXP407 appeared less potent toward purified ACE from rat and furthermore was unable to block ACE activity present in crude rat plasma. This study demonstrated that for further evaluation of the in vivo efficacy of RXP407, mice rather than rats should be used as the animal model. Thus, following the change in the Ac-S-D-K-P plasmatic levels, after i.v. injection of RXP407 to mice, will permit the potency and selectivity of this novel ACE inhibitor to be assessed.
Analysis of the evolution of angiotensin II type 1 receptor gene in mammals (mouse, rat, bovine and human).[Pubmed:1497638]
Biochem Biophys Res Commun. 1992 Jul 31;186(2):1042-9.
The nucleotide and amino acid sequences for mouse angiotensin II (AII) type 1A and 1B receptors were deduced from their complementary and genomic DNAs. Evolutionary analyses based on the nucleotide sequences of the coding region of AII type 1 receptor genes indicated that the duplication event of the type 1 gene occurred 24 +/- 2 million years ago before the divergence between the rat and mouse but after the divergence between rodents and the human/artiodactyls couple. This conclusion was consistent with the results of genomic Southern blot analyses, which revealed that the mouse and rat possess 2 similar but separate genes, whereas the bovine and human have only a single class gene.
Renal metabolism of angiotensin I and II.[Pubmed:2175370]
Kidney Int Suppl. 1990 Nov;30:S24-7.
The enzymatic hydrolysis of angiotensin I and II is reviewed briefly with emphasis on two enzymes, the angiotensin I converting enzyme and neutral endopeptidase 24.11. Angiotensin I is converted to angiotensin II by converting enzyme present in many tissues and highly concentrated in the human kidney and in kidney of some laboratory animals. In addition, there is mounting evidence, collected mostly in experiments in vitro, that other enzymes may be able to activate angiotensin I, for example by the stepwise release of the C-terminal His and Leu residues. Angiotensin I, instead of being activated, could be inactivated by the cleavage of its C-terminal tripeptide either by neutral endopeptidase 24.11 or by prolyl endopeptidase. Angiotensin II is cleaved by several peptidases widely distributed in the kidney. One of the products, des-Phe8-angiotensin II, is not entirely inactive as it has an effect in the CNS.
Endothelium-dependent contractions induced by angiotensin I and angiotensin II in canine cerebral artery.[Pubmed:2795464]
J Pharmacol Exp Ther. 1989 Oct;251(1):317-20.
Whether angiotensin I and angiotensin II caused endothelium-dependent contraction was examined in canine cerebral arteries. In endothelium-intact preparations, angiotensin I and angiotensin II at 10(-8) to 10(-6) M caused dose-dependent contractions, whereas both angiotensins caused much less contractions in endothelium-removed preparations. The contractions induced by angiotensin I and angiotensin II were strongly attenuated by aspirin (cyclooxygenase inhibitor) (5 x 10(-5) M), OKY-046 [thromboxane (TX) A2 synthetase inhibitor] (10(-5) M) and ONO-3708 (TX)A2 antagonist) (5 X 10(-9) M). Captopril (10(-6) M) significantly attenuated the contractions induced by angiotensin I but not those induced by angiotensin II. Angiotensin I- and angiotensin II- induced contractions were inhibited markedly by Sar1, Ala8-angiotensin II (10(-9) and 10(-8) M). The present experiments demonstrate that angiotensin I and angiotensin II produce endothelium-dependent contraction in canine cerebral artery via a factor which appears to be TXA2. Angiotensin I may be converted by endothelial cells to angiotensin II, which may activate the cells to produce TXA2 in canine cerebral artery.
Conversion of angiotensin I to angiotensin II.[Pubmed:190881]
Am J Med. 1976 May 31;60(6):749-59.
The angiotensin I converting enzyme has two important functions: it inactivates bradykinin and converts angiotensin I to angiotensin II. Inhibition of the enzyme blocks the renin-angiotensin system and decreases systemic blood pressure if the pressure is maintained or increased by renin. The enzyme occurs in a variety of tissues and cell forms. The vascular endothelial cells of the lung and of peripheral blood vessels, and the epithelial cells of the kidney tubules are major sources of the enzyme. In addition to inactivating hypotensive peptides and activating a hypertensive one in the systemic circulation, the enzyme may affect organ functions by hydrolyzing peptides that are formed and released locally.


