HeliosupineCAS# 32728-78-2 |
- Echimidine
Catalog No.:BCN1967
CAS No.:520-68-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 32728-78-2 | SDF | Download SDF |
| PubChem ID | 5317993 | Appearance | Colorless viscous oil |
| Formula | C20H31NO7 | M.Wt | 397.47 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Cyanoglossophine | ||
| Solubility | Soluble in chloroform | ||
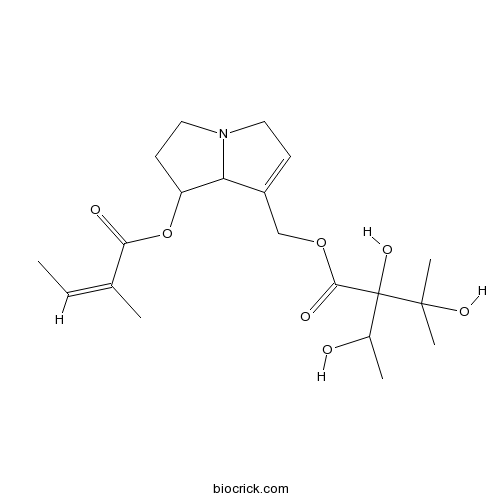
| Chemical Name | [7-[(Z)-2-methylbut-2-enoyl]oxy-5,6,7,8-tetrahydro-3H-pyrrolizin-1-yl]methyl 2,3-dihydroxy-2-(1-hydroxyethyl)-3-methylbutanoate | ||
| SMILES | CC=C(C)C(=O)OC1CCN2C1C(=CC2)COC(=O)C(C(C)O)(C(C)(C)O)O | ||
| Standard InChIKey | HRSGCYGUWHGOPY-SDQBBNPISA-N | ||
| Standard InChI | InChI=1S/C20H31NO7/c1-6-12(2)17(23)28-15-8-10-21-9-7-14(16(15)21)11-27-18(24)20(26,13(3)22)19(4,5)25/h6-7,13,15-16,22,25-26H,8-11H2,1-5H3/b12-6- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Heliosupine, heliosupine N-oxide, 3 ′-O-acetylheliosupine N-oxide,and 7-O- angeloylechinatine N-oxide show inhibition activity against the acetylcholinesterase(AChE), with IC50 0.53-0.60 mM. 2. Heliosupine can deter feeding by the polyphagous larvae of Spodoptera exigua. |
| Targets | AChR | Antifection |

Heliosupine Dilution Calculator

Heliosupine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5159 mL | 12.5796 mL | 25.1591 mL | 50.3183 mL | 62.8978 mL |
| 5 mM | 0.5032 mL | 2.5159 mL | 5.0318 mL | 10.0637 mL | 12.5796 mL |
| 10 mM | 0.2516 mL | 1.258 mL | 2.5159 mL | 5.0318 mL | 6.2898 mL |
| 50 mM | 0.0503 mL | 0.2516 mL | 0.5032 mL | 1.0064 mL | 1.258 mL |
| 100 mM | 0.0252 mL | 0.1258 mL | 0.2516 mL | 0.5032 mL | 0.629 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- tcY-NH2
Catalog No.:BCC5770
CAS No.:327177-34-4
- Cyclohexanecarboxylic acid
Catalog No.:BCN3443
CAS No.:98-89-5
- VU 0285683
Catalog No.:BCC6154
CAS No.:327056-22-4
- TDZD-8
Catalog No.:BCC4258
CAS No.:327036-89-5
- TCS-PIM-1-4a
Catalog No.:BCC5461
CAS No.:327033-36-3
- Chlorogenic acid
Catalog No.:BCN5906
CAS No.:327-97-9
- H-Nle-OH
Catalog No.:BCC3295
CAS No.:327-57-1
- MHY1485
Catalog No.:BCC6404
CAS No.:326914-06-1
- Shz 1
Catalog No.:BCC6334
CAS No.:326886-05-9
- FAAH inhibitor 1
Catalog No.:BCC4254
CAS No.:326866-17-5
- Edpetiline
Catalog No.:BCN6771
CAS No.:32685-93-1
- H-Glu(OtBu)-OtBu.HCl
Catalog No.:BCC2934
CAS No.:32677-01-3
- Phorbol 13-acetate
Catalog No.:BCN7231
CAS No.:32752-29-7
- Macrocarpal L
Catalog No.:BCN5248
CAS No.:327601-97-8
- BIO 5192
Catalog No.:BCC8002
CAS No.:327613-57-0
- Macrocarpal O
Catalog No.:BCN7371
CAS No.:327622-65-1
- Protopanaxatriol
Catalog No.:BCC9245
CAS No.:32773-56-1
- Labetalol HCl
Catalog No.:BCC5489
CAS No.:32780-64-6
- Panaxatriol
Catalog No.:BCN1081
CAS No.:32791-84-7
- Nomifensine
Catalog No.:BCC7226
CAS No.:32795-47-4
- H-D-Leu-OH
Catalog No.:BCC2975
CAS No.:328-38-1
- Ceranib 1
Catalog No.:BCC6186
CAS No.:328076-61-5
- Phortress
Catalog No.:BCC3901
CAS No.:328087-38-3
- Coniferyl alcohol
Catalog No.:BCN4651
CAS No.:32811-40-8
Pyrrolizidine alkaloids from Solenanthus lanatus DC. with acetylcholinesterase inhibitory activity.[Pubmed:26735939]
Nat Prod Res. 2016 Nov;30(22):2567-2574.
The whole plant ethanolic extract of Solenanthus lanatus was used for the isolation of acetylcholinesterase inhibitors. A new pyrrolizidine alkaloid, 7-O-angeloylechinatine N-oxide, 1, was isolated together with three known compounds of the same class (3'-O-acetylHeliosupine N-oxide, 2, Heliosupine N-oxide, 3, and Heliosupine, 4), by bioassay-guided approach. Their structures were elucidated by spectroscopic methods. All the isolated compounds showed inhibition activity against the AChE, with IC50 0.53-0.60 mM.
Phytotoxic and antimicrobial activity of 5,7-dihydroxychromone from peanut shells.[Pubmed:24234013]
J Chem Ecol. 1995 Feb;21(2):107-15.
A flavonoid decomposition product that is present in peanut (Arachis hypogaea) shells, 5,7-dihydroxychromone (DHC), was found to inhibit the radial growth of cultures of the soil pathogenic fungiRhizoctonia solani andSclerotium rolfsii with I50 (the concentrations of DHC required to inhibit growth 50%) values of 18 and 26microM, respectively. Radicle elongation of velvetleaf, corn, peanut, and wheat was inhibited by DHC with I50 values of 30, 50, 65 and 200microM, respectively. DHC had no effect on the growth ofBradyrhizobium sp. at 10microM in medium containing low (1.0 g/liter) mannitol as the carbon source, although the related flavones luteolin and chrysin each promoted bacterial growth at 10microM 48 hr after inoculation. When tested in high (10.0 g/liter) mannitol medium, DHC initially inhibited growth ofBradyrhizobium sp., but 120 hr after inoculation the growth of all treatments were similar. These results suggest a role for DHC released from peanut shells in suppressing pathogenic fungal infection and competing plant growth but not forBradyrhizobium growth promotion.


