NomifensinePotent noradrenalin and dopamine uptake inhibitor. Antidepressant CAS# 32795-47-4 |
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- MMAD
Catalog No.:BCC1774
CAS No.:203849-91-6
- Eribulin
Catalog No.:BCC5174
CAS No.:253128-41-5
- Colchicine
Catalog No.:BCN6271
CAS No.:64-86-8
- Mc-MMAE
Catalog No.:BCC5201
CAS No.:863971-24-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 32795-47-4 | SDF | Download SDF |
| PubChem ID | 5358907 | Appearance | Powder |
| Formula | C20H22N2O4 | M.Wt | 354.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 80 mg/mL (225.73 mM) H2O : 2.2 mg/mL (6.21 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
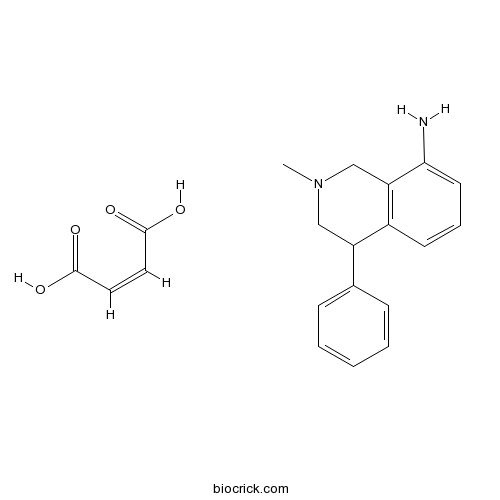
| Chemical Name | (Z)-but-2-enedioic acid;2-methyl-4-phenyl-3,4-dihydro-1H-isoquinolin-8-amine | ||
| SMILES | CN1CC(C2=C(C1)C(=CC=C2)N)C3=CC=CC=C3.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | GEOCVSMCLVIOEV-BTJKTKAUSA-N | ||
| Standard InChI | InChI=1S/C16H18N2.C4H4O4/c1-18-10-14(12-6-3-2-4-7-12)13-8-5-9-16(17)15(13)11-18;5-3(6)1-2-4(7)8/h2-9,14H,10-11,17H2,1H3;1-2H,(H,5,6)(H,7,8)/b;2-1- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent inhibitor of noradrenalin and dopamine uptake (Ki values are 4.7, 26 and 4000 nM for inhibition of noradrenalin, dopamine and 5-HT uptake respectively, in rat brain). Increases central dopamine levels in rats following systemic administration. Displays antidepressant activity. |

Nomifensine Dilution Calculator

Nomifensine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8217 mL | 14.1084 mL | 28.2167 mL | 56.4334 mL | 70.5418 mL |
| 5 mM | 0.5643 mL | 2.8217 mL | 5.6433 mL | 11.2867 mL | 14.1084 mL |
| 10 mM | 0.2822 mL | 1.4108 mL | 2.8217 mL | 5.6433 mL | 7.0542 mL |
| 50 mM | 0.0564 mL | 0.2822 mL | 0.5643 mL | 1.1287 mL | 1.4108 mL |
| 100 mM | 0.0282 mL | 0.1411 mL | 0.2822 mL | 0.5643 mL | 0.7054 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Panaxatriol
Catalog No.:BCN1081
CAS No.:32791-84-7
- Labetalol HCl
Catalog No.:BCC5489
CAS No.:32780-64-6
- Protopanaxatriol
Catalog No.:BCC9245
CAS No.:32773-56-1
- Macrocarpal O
Catalog No.:BCN7371
CAS No.:327622-65-1
- BIO 5192
Catalog No.:BCC8002
CAS No.:327613-57-0
- Macrocarpal L
Catalog No.:BCN5248
CAS No.:327601-97-8
- Phorbol 13-acetate
Catalog No.:BCN7231
CAS No.:32752-29-7
- Heliosupine
Catalog No.:BCN1980
CAS No.:32728-78-2
- tcY-NH2
Catalog No.:BCC5770
CAS No.:327177-34-4
- Cyclohexanecarboxylic acid
Catalog No.:BCN3443
CAS No.:98-89-5
- VU 0285683
Catalog No.:BCC6154
CAS No.:327056-22-4
- TDZD-8
Catalog No.:BCC4258
CAS No.:327036-89-5
- H-D-Leu-OH
Catalog No.:BCC2975
CAS No.:328-38-1
- Ceranib 1
Catalog No.:BCC6186
CAS No.:328076-61-5
- Phortress
Catalog No.:BCC3901
CAS No.:328087-38-3
- Coniferyl alcohol
Catalog No.:BCN4651
CAS No.:32811-40-8
- (H-Cys-OMe)2.2HCl
Catalog No.:BCC2916
CAS No.:32854-09-4
- Lannaconitine
Catalog No.:BCN2504
CAS No.:32854-75-4
- GlyH-101
Catalog No.:BCC4104
CAS No.:328541-79-3
- AG-14361
Catalog No.:BCC2209
CAS No.:328543-09-5
- Benzylamine hydrochloride
Catalog No.:BCN6908
CAS No.:3287-99-8
- Cajanin
Catalog No.:BCN5249
CAS No.:32884-36-9
- Lasiodiplodin
Catalog No.:BCN4770
CAS No.:32885-81-7
- De-O-methyllasiodiplodin
Catalog No.:BCN7187
CAS No.:32885-82-8
Monitoring Dopamine Responses to Potassium Ion and Nomifensine by in Vivo Microdialysis with Online Liquid Chromatography at One-Minute Resolution.[Pubmed:28094974]
ACS Chem Neurosci. 2017 Feb 15;8(2):329-338.
Recently, our laboratory has demonstrated the technical feasibility of monitoring dopamine at 1 min temporal resolution with microdialysis and online liquid chromatography. Here, we monitor dopamine in the rat striatum during local delivery of high potassium/low sodium or Nomifensine in awake-behaving rats. Microdialysis probes were implanted and perfused continuously with or without dexamethasone in the perfusion fluid for 4 days. Dexamethasone is an anti-inflammatory agent that exhibits several positive effects on the apparent health of the brain tissue surrounding microdialysis probes. Dopamine was monitored 1 or 4 days after implantation under basal conditions, during 10 min applications of 60 mM or 100 mM K(+), and during 15 min applications of 10 muM Nomifensine. High K(+) and Nomifensine were delivered locally by adding them to the microdialysis perfusion fluid using a computer-controlled, low-dead-volume six-port valve. Each day/K(+)/dexamethasone combination elicited specific dopamine responses. Dexamethasone treatment increased dopamine levels in basal dialysates (i.e., in the absence of K(+) or Nomifensine). Applications of 60 mM K(+) evoked distinct responses on days one and four after probe implantation, depending upon the presence or absence of dexamethasone, consistent with dexamethasone's ability to mitigate the traumatic effect of probe implantation. Applications of 100 mM K(+) evoked dramatic oscillations in dopamine levels that correlated with changes in the field potential at a metal electrode implanted adjacent to the microdialysis probe. This combination of results indicates the role of spreading depolarization in response to 100 mM K(+). With 1 min temporal resolution, we find that it is possible to characterize the pharmacokinetics of the response to the local delivery of Nomifensine. Overall, the findings reported here confirm the benefits arising from the ability to monitor dopamine via microdialysis at high sensitivity and at high temporal resolution.
Differential effects of nomifensine and imipramine on motivated behavior in the runway model of intracranial self-stimulation.[Pubmed:24436978]
Eur J Pharmacol. 2013 Nov 15;720(1-3):186-191.
A motivational deficit (the loss of pleasure or interest in previously rewarding stimuli) is one of the core symptoms of major depression, and valid models evaluating the motivational effects of drugs are needed. It was recently demonstrated that the priming stimulation effect in the runway model of intracranial self-stimulation (ICSS) can be used as a model system to study the motivational effects of drugs. However, the characteristics of this novel experimental model have not been fully clarified. In this study, we investigated the effects of Nomifensine and imipramine in the runway ICCS model, forced swim tests, and locomotor activity tests to differentiate motivation from affective-like states. Nomifensine dose-dependently increased running speed on the runway and decreased immobility time in the forced swim test. In contrast, imipramine decreased running speed on the runway although it also decreased immobility time in the forced swim test. In addition, the motivation-enhancing effect of nomifesine in the runway model was completely inhibited by pretreatment with the dopamine receptor antagonist haloperidol, although Nomifensine-induced increases in locomotion were not affected by haloperidol. These results demonstrate that Nomifensine displays motivation-enhancing and antidepressant-like effects. In addition, the motivational effects of Nomifensine in the runway ICSS model are primarily mediated by dopamine receptors and enhancements of motivated behavior do not simply reflect hyperlocomotion.
Region- and domain-dependent action of nomifensine.[Pubmed:24766210]
Eur J Neurosci. 2014 Jul;40(2):2320-8.
The dopamine (DA) terminal fields in the rat dorsal striatum (DS) and nucleus accumbens core (NAcc) are organized as patchworks of domains that exhibit distinct kinetics of DA release and clearance. The present study used fast-scan cyclic voltammetry recordings of electrically evoked DA overflow to test the hypothesis that Nomifensine might exhibit domain-dependent actions within the NAcc, as we previously found to be the case within the DS. Within the NAcc, Nomifensine preferentially enhanced evoked DA overflow in the slow domains compared with the fast domains. To seek a kinetic explanation for Nomifensine's selective actions, we quantified the apparent KM of DA clearance by numerically evaluating the derivative of the descending phase of the DA signal after the end of the stimulus. For comparison, we likewise quantified the apparent KM in the domains of the DS. As expected, because it is a competitive inhibitor, Nomifensine significantly increased the apparent KM in both the fast and slow domains of both the NAcc and DS. However, our analysis also led to the novel finding that Nomifensine preferentially increases the apparent KM in the NAcc compared with the DS; the apparent KM increased by ~500% in the NAcc and by ~200% in the DS.
Effects of acute and sustained administration of the catecholamine reuptake inhibitor nomifensine on the firing activity of monoaminergic neurons.[Pubmed:19939862]
J Psychopharmacol. 2010 Aug;24(8):1223-35.
Nomifensine potently inhibits the reuptake of norepinephrine and dopamine in vitro. It is one of few antidepressants with marked potency to block dopamine reuptake that has ever been used clinically. Acute and sustained administration of Nomifensine was investigated on the firing of monoaminergic neurons to understand its mechanism of action. In vivo extracellular recordings of locus coeruleus, ventral tegmental area and dorsal raphe nucleus neurons were obtained from male Sprague-Dawley rats. The intravenous injection of Nomifensine in the locus coeruleus and ventral tegmental area yielded ED(50) values of 40 +/- 1 and 450 +/- 41 microg/kg, respectively, suggesting that Nomifensine directly acted upon dopamine and norepinephrine neurons, since these values are proportional to its affinities for norepinephrine and dopamine transporters. There was no effect on 5-HT neurons. Nomifensine (5 mg/kg/day, subcutaneous, using minipumps) potently and significantly inhibited dopamine neuronal firing in the ventral tegmental area after 2 days, with recovery to normal after the 14-day treatment due to D(2) autoreceptor desensitization. Norepinephrine neuronal firing in the locus coeruleus was significantly decreased after 2 and 14 days. A significant increase in dorsal raphe nucleus 5-HT neuronal firing was seen after a two-day regimen, and remained elevated after 14 days. Desensitization of the 5-HT(1A) receptor on 5-HT neurons of the dorsal raphe nucleus occurred after two days of Nomifensine administration. Nomifensine likely treated depression by acting on dopamine, norepinephrine and 5-HT neurons, highlighting the importance of the functional connectivity between these three monoaminergic systems.
Effects of various dopamine uptake inhibitors on striatal extracellular dopamine levels and behaviours in rats.[Pubmed:7589207]
Eur J Pharmacol. 1995 Aug 4;281(2):195-203.
In vivo central effects of some dopamine uptake inhibitors were evaluated in both brain microdialysis and behavioural studies in rats, and compared with their in vitro affinities to dopamine uptake sites. IC50 values of GBR12909 (1-[2- bis(4-fluorophenyl)methoxy]ethyl]-4-(3- phenylpropyl)piperazine), diclofensine, mazindol, amfonelic acid and Nomifensine for inhibiting 1 nM [3H]GBR12935 (1-[2-(diphenylmethoxy)ethyl]-4-(3-phenylpropyl)piperazine) binding to rat striatal membrane were 7.0, 36, 81, 187 and 290 nM, respectively. In the brain microdialysis study, dopamine levels in the striatal dialysates were increased to 16.3- (GBR12909), 14.1- (Nomifensine), 4.8- (diclofensine) and 1.9-fold (amfonelic acid) the respective basal levels 40-60 min after i.p. administration (0.1 mmol/kg) and thereafter decreased slowly but remained at the elevated levels for a further 3 h, while mazindol gradually increased dopamine levels though less pronouncedly than others (1.7-fold 200 min after administration). Remarkable and comparable stereotyped behaviours (licking and forepaw treading) were continuously observed at least for 3 h after administration of GBR12909, Nomifensine and amfonelic acid, while stereotypies induced by diclofensine and mazindol were moderate and marginal, respectively. In vivo potencies of dopamine uptake inhibitors to increase the extracellular dopamine levels in the striatum tended to correlate with their in vitro affinities to dopamine uptake sites except in the case of Nomifensine, and correlated significantly with their potencies to induce stereotyped behaviours except in the case of amfonelic acid. Based on these findings, pharmacological characteristics of these dopamine uptake inhibitors are discussed.
Nomifensine and its derivatives as possible tools for studying amine uptake.[Pubmed:844493]
Eur J Pharmacol. 1977 Mar 21;42(2):101-6.
An experimental antidepressive drug, Nomifensine, was tested in simultaneous experiments as an inhibitor of the uptake of noradrenaline (NA), dopamine (DA) and 5-hydroxytryptamine (5-HT) in rat brain synaptosomes. The drug was found to be a very potent inhibitor of NA (Ki 4.7 X 10(-9) M) and DA (Ki 2.6 X 10(-8) M) uptake, but relatively weak inhibitor of 5-HT uptake (Ki appr. 4 X 10(-6) M). According to kinetic studies on dopamine uptake, the inhibition was competitive. Time course studies indicated that the percentage inhibition did not increase with time. This finding suggests that inhibition of membrane uptake rather than inhibition of storage is the mechanism of action of this drug. 3 metabolites of Nomifensine were also tested as inhibitors of NA and DA uptake. The addition of a 4-hydroxy group to the phenyl ring of Nomifensine slightly decreased the potency, and addition of hydroxy and methoxy groups to the positions 3 and 4 in the phenyl ring, clearly decreased the potency. The structure of Nomifensine is compared to that of chlorimipramine. It is suggested that the differences in selectivity as to dopamine and 5-HT uptake mechanisms might be due to 2 conformational differences: one of the phenyl rings is freely rotating in Nomifensine but not in chlorimipramine, and the tertiary amine group is in a flexible side chain in chlorimipramine but rigidly tied in Nomifensine.


