Gancaonin GCAS# 126716-34-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 126716-34-5 | SDF | Download SDF |
| PubChem ID | 480780 | Appearance | Powder |
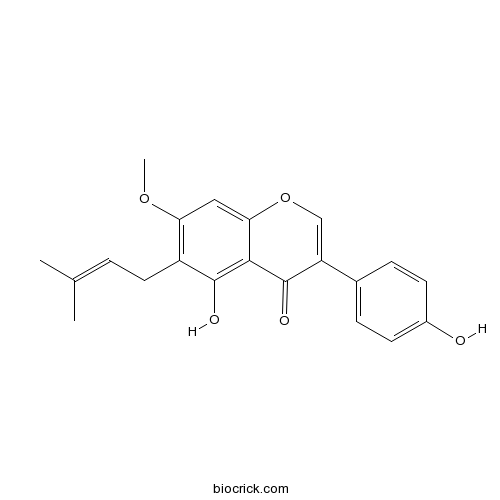
| Formula | C21H20O5 | M.Wt | 352.39 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-hydroxy-3-(4-hydroxyphenyl)-7-methoxy-6-(3-methylbut-2-enyl)chromen-4-one | ||
| SMILES | CC(=CCC1=C(C=C2C(=C1O)C(=O)C(=CO2)C3=CC=C(C=C3)O)OC)C | ||
| Standard InChIKey | WLPHLDLTTPUDSI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H20O5/c1-12(2)4-9-15-17(25-3)10-18-19(20(15)23)21(24)16(11-26-18)13-5-7-14(22)8-6-13/h4-8,10-11,22-23H,9H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Gancaonin G shows more moderate antibacterial activity against Streptococcus mutans. 2. Gancaonin G shows antibacterial effects on the MRSA strains with MIC values of 16 microg/ml. |
| Targets | Antifection |

Gancaonin G Dilution Calculator

Gancaonin G Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8378 mL | 14.1888 mL | 28.3776 mL | 56.7553 mL | 70.9441 mL |
| 5 mM | 0.5676 mL | 2.8378 mL | 5.6755 mL | 11.3511 mL | 14.1888 mL |
| 10 mM | 0.2838 mL | 1.4189 mL | 2.8378 mL | 5.6755 mL | 7.0944 mL |
| 50 mM | 0.0568 mL | 0.2838 mL | 0.5676 mL | 1.1351 mL | 1.4189 mL |
| 100 mM | 0.0284 mL | 0.1419 mL | 0.2838 mL | 0.5676 mL | 0.7094 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isoaltenuene
Catalog No.:BCN7313
CAS No.:126671-80-5
- UTPγS trisodium salt
Catalog No.:BCC7624
CAS No.:1266569-94-1
- KT 5823
Catalog No.:BCC7006
CAS No.:126643-37-6
- 16-Epinormacusine B
Catalog No.:BCN4030
CAS No.:126640-98-0
- A 887826
Catalog No.:BCC7898
CAS No.:1266212-81-0
- Trilobinine
Catalog No.:BCN7927
CAS No.:126595-92-4
- Vallesamine N-oxide
Catalog No.:BCN6144
CAS No.:126594-73-8
- Cyclocerberidol
Catalog No.:BCN6143
CAS No.:126594-66-9
- Cerberidol
Catalog No.:BCN6142
CAS No.:126594-64-7
- S1RA hydrochloride
Catalog No.:BCC4190
CAS No.:1265917-14-3
- 8,14-Epoxyergosta-4,22-diene-3,6-dione
Catalog No.:BCN1591
CAS No.:1265908-20-0
- Ciclesonide
Catalog No.:BCC5234
CAS No.:126544-47-6
- Gancaonin I
Catalog No.:BCN7144
CAS No.:126716-36-7
- Tilifodiolide
Catalog No.:BCN6145
CAS No.:126724-95-6
- Acetylsventenic acid
Catalog No.:BCN4849
CAS No.:126737-42-6
- Sarafotoxin S6a
Catalog No.:BCC5834
CAS No.:126738-34-9
- GR 89696 fumarate
Catalog No.:BCC7083
CAS No.:126766-32-3
- Sventenic acid
Catalog No.:BCN3923
CAS No.:126778-79-8
- Ulipristal acetate
Catalog No.:BCC4068
CAS No.:126784-99-4
- 5,7,3'-Trihydroxy-4'-methoxy-8-prenylflavanone
Catalog No.:BCN1590
CAS No.:1268140-15-3
- 13-Acetoxy-3beta-hydroxygermacra-1(10)E,4E,7(11)-trien-12,6alpha-olide
Catalog No.:BCN7314
CAS No.:126829-66-1
- 8alpha-Acetoxyarglabin
Catalog No.:BCN7315
CAS No.:126829-70-7
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- (-)-JQ1
Catalog No.:BCC3603
CAS No.:1268524-71-5
Phenolic constituents of licorice. VIII. Structures of glicophenone and glicoisoflavanone, and effects of licorice phenolics on methicillin-resistant Staphylococcus aureus.[Pubmed:10993226]
Chem Pharm Bull (Tokyo). 2000 Sep;48(9):1286-92.
Two new phenolic compounds, glicophenone (1) and glicoisoflavanone (2), were isolated from commercial licorice, and their structures were elucidated on the basis of spectroscopic data. Antibacterial assays of licorice phenolics for Staphylococcus aureus, including four strains of methicillin-resistant S. aureus (MRSA), and also for Escherichia coli K12 and Pseudomonas aeruginosa PAO1, were then examined. Two compounds among them, 8-(gamma,gamma-dimethylallyl)-wighteone (21) and 3'-(gamma,gamma-dimethylallyl)-kievitone (28), showed remarkable antibacterial effects [minimum inhibitory concentrations (MICs), 8 microg/ml on the MRSA strains and methicillin-sensitive S. aureus. Licochalcone A (14), Gancaonin G (20), isoangustone A (24), glyasperins C (30) and D (31), glabridin, (32), licoricidin (33), glycycoumarin (34) and licocoumarone (40) showed antibacterial effects on the MRSA strains with MIC values of 16 microg/ml. Effects on the beta-lactam resistance of the MRSA strains were also examined, and licoricidin (33) noticeably decreased the resistance of the MRSA strains against oxacillin, as shown by the reduction in the MICs of oxacillin (lower than 1/128-1/1000 in the presence of 8 microg/ml of 33, and 1/8-1/32 in the presence of 4 microg/ml of 33). Mechanistic study suggested that 33 does not inhibit the formation of penicillin-binding protein 2' (PBP2'), but affects the enzymatic function of PBP2'.
Pyrano-isoflavans from Glycyrrhiza uralensis with antibacterial activity against Streptococcus mutans and Porphyromonas gingivalis.[Pubmed:24479468]
J Nat Prod. 2014 Mar 28;77(3):521-6.
Continuing investigation of fractions from a supercritical fluid extract of Chinese licorice (Glycyrrhiza uralensis) roots has led to the isolation of 12 phenolic compounds, of which seven were described previously from this extract. In addition to these seven metabolites, four known components, 1-methoxyerythrabyssin II (4), 6,8-diprenylgenistein, Gancaonin G (5), and isoglycyrol (6), and one new isoflavan, licorisoflavan C (7), were characterized from this material for the first time. Treatment of licoricidin (1) with palladium chloride afforded larger amounts of 7 and also yielded two new isoflavans, licorisoflavan D (8), which was subsequently detected in the licorice extract, and licorisoflavan E (9). Compounds 1-9 were evaluated for their antibacterial activities against the cariogenic Streptococcus mutans and the periodontopathogenic Porphyromonas gingivalis. Licoricidin (1), licorisoflavan A (2), and 7-9 showed antibacterial activity against P. gingivalis (MICs of 1.56-12.5 mug/mL). The most potent activity against S. mutans was obtained with 7 (MIC of 6.25 mug/mL), followed by 1 and 9 (MIC of 12.5 mug/mL). This study provides further evidence for the therapeutic potential of licorice extracts for the treatment and prevention of oral infections.
Antibacterial compounds from Glycyrrhiza uralensis.[Pubmed:16441081]
J Nat Prod. 2006 Jan;69(1):121-4.
From the roots of Glycyrrhiza uralensis, two new pterocarpenes, glycyrrhizol A (1) and glycyrrhizol B (2), along with four known isoflavonoids, 5-O-methylglycryol (3), isoglycyrol (4), 6,8-diisoprenyl-5,7,4'-trihydroxyisoflavone (5), and Gancaonin G (6), were isolated using a bioassay-guided fractionation method. The structures of the new compounds (1and 2) were elucidated by spectroscopic data interpretation. The known compounds (3-6) were identified by comparison of their spectroscopic data with reported values in the literature. Glycyrrhizol A (1) and 6,8-diisoprenyl-5,7,4'-trihydroxyisoflavone (5) exhibited potent antibacterial activity against Streptococcus mutans with minimum inhibitory concentrations of 1 and 2 microg/mL, respectively, while glycyrrhizol B (2) and Gancaonin G (6) showed more moderate activity.


