Sarafotoxin S6aEndothelin receptor agonist CAS# 126738-34-9 |
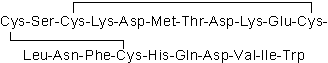
- A 419259 trihydrochloride
Catalog No.:BCC4308
CAS No.:1435934-25-0
- PP 2 (AG 1879)
Catalog No.:BCC3631
CAS No.:172889-27-9
- PD173955
Catalog No.:BCC3999
CAS No.:260415-63-2
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- WH-4-023
Catalog No.:BCC8051
CAS No.:837422-57-8
- KX2-391
Catalog No.:BCC5080
CAS No.:897016-82-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 126738-34-9 | SDF | Download SDF |
| PubChem ID | 71308664 | Appearance | Powder |
| Formula | C105H156N28O34S5 | M.Wt | 2514.85 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
| Sequence | CSCKDMTDKECLNFCHQDVIW (Modifications: Disulfide bridge between 1 - 15, 3 - 11) | ||
| SMILES | CCC(C)C(C(=O)NC(CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)C(C(C)C)NC(=O)C(CC(=O)O)NC(=O)C(CCC(=O)N)NC(=O)C(CC3=CNC=N3)NC(=O)C4CSSCC(C(=O)NC(C(=O)NC5CSSCC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N4)CC6=CC=CC=C6)CC(=O)N)CC(C)C)NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC5=O)CCCCN)CC(=O)O)CCSC)C(C)O)CC(=O)O)CCCCN)CCC(=O)O)CO)N | ||
| Standard InChIKey | BOQKWORWRAYXSI-SQCNKGOOSA-N | ||
| Standard InChI | InChI=1S/C105H156N28O34S5/c1-9-51(6)83(103(164)126-71(105(166)167)35-54-41-112-58-22-14-13-21-56(54)58)132-102(163)82(50(4)5)131-97(158)70(40-81(144)145)124-88(149)61(25-27-76(109)136)117-93(154)66(36-55-42-111-48-113-55)121-101(162)75-45-170-169-44-57(108)85(146)127-72(43-134)98(159)130-73-46-171-172-47-74(100(161)119-64(33-49(2)3)91(152)122-67(37-77(110)137)94(155)120-65(92(153)129-75)34-53-19-11-10-12-20-53)128-89(150)62(26-28-78(138)139)116-86(147)59(23-15-17-30-106)114-96(157)69(39-80(142)143)125-104(165)84(52(7)135)133-90(151)63(29-32-168-8)118-95(156)68(38-79(140)141)123-87(148)60(115-99(73)160)24-16-18-31-107/h10-14,19-22,41-42,48-52,57,59-75,82-84,112,134-135H,9,15-18,23-40,43-47,106-108H2,1-8H3,(H2,109,136)(H2,110,137)(H,111,113)(H,114,157)(H,115,160)(H,116,147)(H,117,154)(H,118,156)(H,119,161)(H,120,155)(H,121,162)(H,122,152)(H,123,148)(H,124,149)(H,125,165)(H,126,164)(H,127,146)(H,128,150)(H,129,153)(H,130,159)(H,131,158)(H,132,163)(H,133,151)(H,138,139)(H,140,141)(H,142,143)(H,144,145)(H,166,167)/t51-,52+,57-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,82-,83-,84-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endothelin receptor agonist (EC50 values are 7.5 and > 150 nM for contraction of pig coronary artery and guinea pig aorta respectively). Nociceptive in vivo. |

Sarafotoxin S6a Dilution Calculator

Sarafotoxin S6a Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Acetylsventenic acid
Catalog No.:BCN4849
CAS No.:126737-42-6
- Tilifodiolide
Catalog No.:BCN6145
CAS No.:126724-95-6
- Gancaonin I
Catalog No.:BCN7144
CAS No.:126716-36-7
- Gancaonin G
Catalog No.:BCN6837
CAS No.:126716-34-5
- Isoaltenuene
Catalog No.:BCN7313
CAS No.:126671-80-5
- UTPγS trisodium salt
Catalog No.:BCC7624
CAS No.:1266569-94-1
- KT 5823
Catalog No.:BCC7006
CAS No.:126643-37-6
- 16-Epinormacusine B
Catalog No.:BCN4030
CAS No.:126640-98-0
- A 887826
Catalog No.:BCC7898
CAS No.:1266212-81-0
- Trilobinine
Catalog No.:BCN7927
CAS No.:126595-92-4
- Vallesamine N-oxide
Catalog No.:BCN6144
CAS No.:126594-73-8
- Cyclocerberidol
Catalog No.:BCN6143
CAS No.:126594-66-9
- GR 89696 fumarate
Catalog No.:BCC7083
CAS No.:126766-32-3
- Sventenic acid
Catalog No.:BCN3923
CAS No.:126778-79-8
- Ulipristal acetate
Catalog No.:BCC4068
CAS No.:126784-99-4
- 5,7,3'-Trihydroxy-4'-methoxy-8-prenylflavanone
Catalog No.:BCN1590
CAS No.:1268140-15-3
- 13-Acetoxy-3beta-hydroxygermacra-1(10)E,4E,7(11)-trien-12,6alpha-olide
Catalog No.:BCN7314
CAS No.:126829-66-1
- 8alpha-Acetoxyarglabin
Catalog No.:BCN7315
CAS No.:126829-70-7
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- (-)-JQ1
Catalog No.:BCC3603
CAS No.:1268524-71-5
- MC 1046
Catalog No.:BCC1733
CAS No.:126860-83-1
- Ssioriside
Catalog No.:BCN6146
CAS No.:126882-53-9
- 3-Methoxy-5-heneicosylphenol
Catalog No.:BCN6147
CAS No.:126882-76-6
- MK 1903
Catalog No.:BCC6242
CAS No.:1268882-43-4
Sarafotoxin S6c is a relatively weak displacer of specifically bound 125I-endothelin.[Pubmed:2543414]
Biochem Biophys Res Commun. 1989 May 30;161(1):89-94.
Sarafotoxin S6a, S6b and S6c are chemically related vasoconstrictor polypeptides obtained from the venom of the snake, Atractaspis engaddensis. Each contains twenty one amino acid residues, two intrachain cysteine linkages and a long hydrophobic tail. Structurally these polypeptides resemble endothelin. Binding studies with 125I-endothelin showed that 125I-endothelin bound to rat ventricular membranes is totally displaceable by sarafotoxin S6b and endothelin, with IC50 values of 0.21 and 0.16 nM, respectively. Sarafotoxin S6c, which differs from sarafotoxin S6b in containing threonine instead of serine at residue 2, arginine instead of lysine at residue 4, and glutamic acid instead of lysine at residue 9, only weakly displaced bound 125I-endothelin (IC50, 854 nM). These results indicate that the ability of the sarafotoxins to interact with the endothelin binding site is not solely dependent on the long hydrophobic tail or the cysteine linkages.
Endothelin-induced nociception in mice: mediation by ETA and ETB receptors.[Pubmed:8632332]
J Pharmacol Exp Ther. 1996 Feb;276(2):647-51.
Endothelins (ET-1, ET-2 or ET-3) or endothelin precursors (big-ET-1[1-38], big-ET-2[1-37] or big-ET-3[1-41]) injected i.p. in mice have previously been shown to elicit a characteristic nociceptive behavioral response. In this study, we investigated the endothelin receptor type (ETA or ETB) that mediates this behavioral response. Mice were injected i.p. with ET-1, ET-2, ET-3, big-ET-1[1-38], big-ET-2[1-37], big-ET-3[1-41], Sarafotoxin S6a, sarafotoxin S6b, sarafotoxin S6c, ET-1 with Ala substitutions for Cys3 and Cys11 or His-Leu-Asp-Ile-Ile-Trp, and quantal dose-response curves were obtained for each of the compounds (except the latter). Co-administration of enzyme inhibitors with the big-endothelins was used to establish the requisite conversion to endothelins and big-ET-1[22-38], big-ET-2[22-37] and ET-3[22-41] amide, and the ETA-selective antagonist cyclo[-D-Asp-Pro-D-Val-Leu-D-Trp-] was used to determine receptor specificity. The ED50 values were 2.9, 3.3 and 23.9 micrograms/kg i.p. for ET-1, ET-2 and ET-3, respectively, 0.6, 0.6 and 13.1 micrograms/kg i.p. for Sarafotoxin S6a, sarafotoxin S6b and sarafotoxin S6c, respectively, and 5.3 micrograms/kg i.p. for ET-1 with Ala substitutions for Cys3 and Cys11. Big-ET-1[22-38], big-ET-2[22-37], big-ET-3[22-41] amide and ET-C produced less than 25% effect up to 2000 micrograms/kg. The big-ET-1-induced effects were blocked by the enzyme inhibitors phosphoramidon and thiorphan (ID50 = 0.9 mg/kg) but not by ubenimex (bestatin), captopril or perindopril. Cyclo[-D-Asp-Pro-D-Val-Leu-D-Trp-] blocked ET-1- and ET-2-induced effects but not ET-3-, ACh- or phenyl-p-quinone-induced effects. These results suggest that endothelin-induced nociceptive behavioral response in mice can be mediated via both ET receptor types, ETA and ETB. Further, the ET-1 carboxy-terminal hexapeptide is insufficient to produce the effect, and the Cys3-Cys11 disulfide bridge of ET-1 is not required.
Differences between endothelin receptors mediating contraction of guinea-pig aorta and pig coronary artery.[Pubmed:8287901]
Eur J Pharmacol. 1993 Nov 9;249(2):199-206.
Endothelin receptors mediating contraction were characterized and compared in rings from guinea-pig thoracic aorta and pig left circumflex coronary artery. In guinea-pig aorta, the following rank order of agonist potencies was found (mean EC50 value, nM): endothelin-1 (5.0) = endothelin-2 (5.5) > vasoactive intestinal contractor (VIC; 11.0) > sarafotoxin S6b (39.8) > [Ala3,11]endothelin-1 (121) > Sarafotoxin S6a (> 150) > endothelin-3 (> 500). [Ala1,3,11,15] Endothelin-1, endothelin-(16-21), sarafotoxin S6c and sarafotoxin S6d were neither agonists nor antagonists at concentrations up to 1, 10, 3 and 1 microM, respectively. Cyclo-(D-Trp-D-Asp-Pro-D-Val-Leu) (BQ-123; 0.1-1 microM) behaved as a competitive antagonist of endothelin-1 (pA2 7.4 +/- 0.1, slope factor 0.91 +/- 0.17, n = 4). In pig coronary artery, all endothelins and sarafotoxins were agonists, except for endothelin-(16-21). Sarafotoxin S6c, [Lys4]sarafotoxin S6c, [Nle6]sarafotoxin S6c and [Ala1,3,11,15]endothelin-1 acted as partial agonists (Emax about 40% of that of endothelin-1). The rank order of agonist potencies was: sarafotoxin S6c (1.5) = [Lys4]sarafotoxin S6c (1.5) > [Nle6]sarafotoxin S6c (6.7) > or = Sarafotoxin S6a (7.5) > or = endothelin-1 (12.6) > or = sarafotoxin S6b (14.8) > or = VIC (18.3) = endothelin-2 (19.3) > or = [Ala1,3,11,15]endothelin-1 (41.7) > or = [Ala3,11]endothelin-1 (55.2) > endothelin-3 (96.8) > sarafotoxin S6d (> 200). Endothelin-(16-21) was neither agonist nor antagonist at 10 microM. The concentration-response curves of endothelin-3 and Sarafotoxin S6a were biphasic, consisting of a higher sensitivity (40-45% of the total effect) and a lower sensitivity component. BQ-123 (0.1-1 microM) did not alter the concentration-response curve of endothelin-1.(ABSTRACT TRUNCATED AT 250 WORDS)
Comparison of responses to sarafotoxins 6a and 6c in pulmonary and systemic vascular beds.[Pubmed:1348398]
Am J Physiol. 1992 Mar;262(3 Pt 2):H852-61.
Cardiovascular and pulmonary responses to sarafotoxin (S) 6a and S6c were investigated in the anesthetized cat. Intravenous injections of the peptides in doses of 0.1-1.0 nmol/kg caused decreases or biphasic changes in arterial pressure (AP) and increases in central venous pressure, pulmonary arterial pressure (PAP), and cardiac output (CO). Secondary decreases in CO were observed in response to higher doses, and biphasic changes in systemic (SVR) and pulmonary (PVR) vascular resistances were observed. Under constant-flow conditions, the peptides only increased pulmonary lobar arterial perfusion pressure and lobar vascular resistance. AP responses to S6a, S6c, endothelin (ET)-1, ET-2, vasoactive intestinal contractor (VIC), and Lys7-ET-1 were similar, whereas AP responses to S6b and ET-3 were similar. S6a, S6b, S6c, ET-1, ET-2, ET-3, VIC, Lys7-ET-1, and big ET-1 increased PAP. S6a and S6c increased distal aortic and superior mesenteric arterial (SMA) blood flow and caused biphasic changes at the highest doses. Under constant-flow conditions, S6a and S6c produced dose-dependent biphasic changes in hindquarters perfusion pressure. Changes in SVR and PVR in response to the peptide were not affected by hexamethonium, glyburide, or meclofenamate, indicating that responses are independent of autonomic reflexes, activation of ATP-regulated K+ channels, or release of cyclooxygenase products. In contrast, N-nitro-L-arginine methyl ester decreased hindquarters vasodilator response to S6a and S6c. The present data show that S6a and S6c produce both vasodilation and vasoconstriction in the systemic vascular bed and increase lobar vascular resistance and that hindquarters vasodilator responses are mediated, in part, by the release of endothelium-derived relaxing factor.


