Ulipristal acetateCAS# 126784-99-4 |
- AR-C155858
Catalog No.:BCC1367
CAS No.:496791-37-8
Quality Control & MSDS
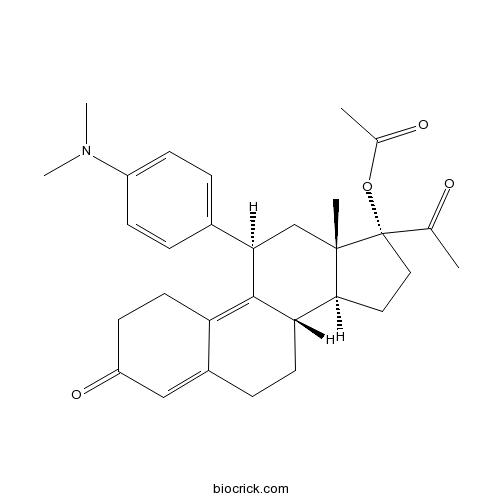
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 126784-99-4 | SDF | Download SDF |
| PubChem ID | 130904 | Appearance | Powder |
| Formula | C30H37NO4 | M.Wt | 475.62 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CDB-2914 | ||
| Solubility | DMSO : 33.33 mg/mL (70.08 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | [(8S,11R,13S,14S,17R)-17-acetyl-11-[4-(dimethylamino)phenyl]-13-methyl-3-oxo-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-17-yl] acetate | ||
| SMILES | CC(=O)C1(CCC2C1(CC(C3=C4CCC(=O)C=C4CCC23)C5=CC=C(C=C5)N(C)C)C)OC(=O)C | ||
| Standard InChIKey | OOLLAFOLCSJHRE-ZHAKMVSLSA-N | ||
| Standard InChI | InChI=1S/C30H37NO4/c1-18(32)30(35-19(2)33)15-14-27-25-12-8-21-16-23(34)11-13-24(21)28(25)26(17-29(27,30)3)20-6-9-22(10-7-20)31(4)5/h6-7,9-10,16,25-27H,8,11-15,17H2,1-5H3/t25-,26+,27-,29-,30-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ulipristal (acetate) is a novel selective progesterone receptor modulator (SPRM) for the treatment of benign gynecological conditions such as uterine myoma.In Vitro:Ulipristal acetate blocks activin A modulation of fibronectin and vascular endothelial growth factor A (VEGF-A) mRNA expression in cultured myometrial and leiomyoma cells[2]. Ulipristal acetate decreases the DNA fragmentation at the 100-ng/mL dose and continuing up to the 10,000-ng/mL dose compared to those spermatozoa in the control group[3].In Vivo:Ulipristal and CDB-4124 have significant antiprogestational activity in vivo[1]. Ulipristal acetate decreases incidences of fibroadenomas and adenocarcinomas in the mammary gland in all treated groups. Ulipristal acetate exposure [AUC(0-24h)] at the highest dose in rats is 67 times human therapeutic exposure at 10 mg/day. In mice, no tumor of any type increases at Ulipristal acetate exposures up to 313 times of therapeutic exposure. Ulipristal acetate-related findings in mice are limited to organ weight changes in the liver, pituitary, thyroid/parathyroid glands, and epididymis as well as minimal panlobular hepatocellular hypertrophy in male and female mice receiving 130 mg/kg/day[4]. Ulipristal acetate (1 mg/kg and 5 mg/kg) increases the frequency with which pathologists assessed the endometrium as being thickened compared to controls in a dose-dependent manner. There is a slight decrease in secretory differentiation with increasing dose of Ulipristal acetate, with small decreases in frequency of sub- and supra-nuclear vacuolation[5]. References: | |||||

Ulipristal acetate Dilution Calculator

Ulipristal acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1025 mL | 10.5126 mL | 21.0252 mL | 42.0504 mL | 52.563 mL |
| 5 mM | 0.4205 mL | 2.1025 mL | 4.205 mL | 8.4101 mL | 10.5126 mL |
| 10 mM | 0.2103 mL | 1.0513 mL | 2.1025 mL | 4.205 mL | 5.2563 mL |
| 50 mM | 0.0421 mL | 0.2103 mL | 0.4205 mL | 0.841 mL | 1.0513 mL |
| 100 mM | 0.021 mL | 0.1051 mL | 0.2103 mL | 0.4205 mL | 0.5256 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ulipristal acetate is a novel selective progesterone receptor modulator (SPRM) for the treatment of benign gynecological conditions such as uterine myoma.
- Sventenic acid
Catalog No.:BCN3923
CAS No.:126778-79-8
- GR 89696 fumarate
Catalog No.:BCC7083
CAS No.:126766-32-3
- Sarafotoxin S6a
Catalog No.:BCC5834
CAS No.:126738-34-9
- Acetylsventenic acid
Catalog No.:BCN4849
CAS No.:126737-42-6
- Tilifodiolide
Catalog No.:BCN6145
CAS No.:126724-95-6
- Gancaonin I
Catalog No.:BCN7144
CAS No.:126716-36-7
- Gancaonin G
Catalog No.:BCN6837
CAS No.:126716-34-5
- Isoaltenuene
Catalog No.:BCN7313
CAS No.:126671-80-5
- UTPγS trisodium salt
Catalog No.:BCC7624
CAS No.:1266569-94-1
- KT 5823
Catalog No.:BCC7006
CAS No.:126643-37-6
- 16-Epinormacusine B
Catalog No.:BCN4030
CAS No.:126640-98-0
- A 887826
Catalog No.:BCC7898
CAS No.:1266212-81-0
- 5,7,3'-Trihydroxy-4'-methoxy-8-prenylflavanone
Catalog No.:BCN1590
CAS No.:1268140-15-3
- 13-Acetoxy-3beta-hydroxygermacra-1(10)E,4E,7(11)-trien-12,6alpha-olide
Catalog No.:BCN7314
CAS No.:126829-66-1
- 8alpha-Acetoxyarglabin
Catalog No.:BCN7315
CAS No.:126829-70-7
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- (-)-JQ1
Catalog No.:BCC3603
CAS No.:1268524-71-5
- MC 1046
Catalog No.:BCC1733
CAS No.:126860-83-1
- Ssioriside
Catalog No.:BCN6146
CAS No.:126882-53-9
- 3-Methoxy-5-heneicosylphenol
Catalog No.:BCN6147
CAS No.:126882-76-6
- MK 1903
Catalog No.:BCC6242
CAS No.:1268882-43-4
- LGX818
Catalog No.:BCC4184
CAS No.:1269440-17-6
- Sar-[D-Phe8]-des-Arg9-Bradykinin
Catalog No.:BCC5996
CAS No.:126959-88-4
- Methylenedihydrotanshinquinone
Catalog No.:BCN3221
CAS No.:126979-81-5
Pregnancy Outcomes Following Ulipristal Acetate Emergency Contraception Failure: A Report of Five Cases.[Pubmed:28368774]
Fetal Pediatr Pathol. 2017 Jun;36(3):213-219.
INTRODUCTION: The emergency contraceptive Ulipristal acetate (UPA) 30 mg is increasingly used by women, but there is no published data on UPA exposure in pregnancy. CASE REPORT: Here we describe five cases of unintended pregnancies following the use of UPA for emergency contraception. Of five pregnant women exposed to UPA, one decided to terminate the pregnancy for personal reasons. Two of them experienced premature rupture of membranes and the babies were born large for gestational age (LGA). The other two women experienced gestational diabetes, and one of them also delivered a LGA baby. The blood glucose levels of the mothers were normal after delivery and at six weeks postpartum. No birth defects and no growth or developmental abnormalities for the infants were reported during 6 months follow-up. CONCLUSION: Pregnant women inadvertently exposed to UPA should be monitored carefully, unless further data are available.
Ulipristal acetate before high complexity endoscopic (hysteroscopic, laparoscopic) myomectomy - a mini-review.[Pubmed:28250723]
Prz Menopauzalny. 2016 Dec;15(4):202-204.
Uterine myomas (fibromas, leiomyomas) are the most common tumours in women, and their clinical signs and symptoms are presented by 25-40% of patients with these benign tumours. According to current guidelines, the armamentarium for myoma management consists of: medical therapy (GnRH, SPRMs), non-surgical alternatives including uterine artery embolisation (UAE), vaginal temporary occlusion of uterine arteries using clamp-like device or MRgFUS technique, and surgical treatment (including minimally invasive techniques). In cases of submucous myomas STEPW classification correlates very well with the risk of incomplete hysteroscopic myomectomies. According to limited literature data, Ulipristal acetate as a pre-treatment seems to be very prudent in high complexity hysteroscopic myomectomy (STEPW II, score 5-6). In patients with large uterine myomas (FIGO type 3, 4, 5) undergoing laparoscopic myomectomy, three-month pre-treatment with Ulipristal acetate before laparoscopy is feasible and can be recommended because of shorter time of surgery, lower intraoperative blood loss, lower haemoglobin drop, and low postoperative blood transfusion rate.
Safety after extended repeated use of ulipristal acetate for uterine fibroids.[Pubmed:28267814]
PLoS One. 2017 Mar 7;12(3):e0173523.
OBJECTIVE: To assess long term safety of extended repeated 3-month courses of Ulipristal acetate (UPA) 10 mg/day, for up to 8 courses, with focus on endometrial and laboratory safety parameters. METHODS: This long-term, multi-center, open-label cohort, follow up study consisted of up to 8 consecutive 3-month courses of daily UPA 10 mg, each separated by a drug free period of 2 spontaneous menstrual bleeds. Sixty-four pre-menopausal women, with moderate to severe symptomatic uterine myoma(s) and heavy bleeding were enrolled and were studied for approximately 4 years. The main outcome measures were endometrial histology, laboratory parameters and general safety. RESULTS: All data was reported in a descriptive manner with no formal statistical comparisons. In the 64 women, non-physiological changes (mostly cyst formation, epithelial and vascular changes) in endometrial histology at screening and after treatment courses 4 and 8 were observed in 18.0%, 21.4% and 16.3% of biopsies, respectively. After treatment cessation, such changes were observed in 9.1% of biopsies. All endometrial biopsies were benign after course 8. The median endometrial thickness was 7.0 mm, 10-18 days after the start of menses following treatment courses 5-8, compared to 9.0 mm at screening (before UPA treatment). No changes in the number and type of laboratory results outside the normal ranges were observed with the increasing treatment courses. In total, adverse events were reported in 10 (16%), 12 (19%), 8 (14%) and 5 (9%) subjects, during treatment courses 5, 6, 7 and 8, respectively of which the most frequent adverse events were headache and hot flush. CONCLUSION: The results of this study further support the safety profile of extended repeated 3 months treatment of symptomatic fibroids with Ulipristal acetate 10 mg/day. Repeated UPA treatment courses did not result in any changes of concern in endometrial histology, endometrial thickness, or laboratory safety measures.
Does Ulipristal Acetate Affect Surgical Experience at Laparoscopic Myomectomy?[Pubmed:28351762]
J Minim Invasive Gynecol. 2017 Jul - Aug;24(5):797-802.
STUDY OBJECTIVE: To compare surgical experience of laparoscopic/robotic myomectomy in premenopausal patients pretreated with Ulipristal acetate (UPA) with women not hormonally pretreated. DESIGN: A retrospective, multicenter cohort study of laparoscopic/robotic myomectomy procedure videos (Canadian Task Force Classification III). SETTING: Multiple university-affiliated tertiary care hospitals. PATIENTS: Fifty-five premenopausal women who underwent laparoscopic/robotic myomectomy for intramural myomas and were either pretreated with 3 months of UPA or had no hormonal pretreatment. INTERVENTIONS: Laparoscopic/robotic myomectomy surgical videos were independently reviewed by 2 gynecologists blinded to whether or not patients received pretreatment with UPA. Each procedure was scored using a novel 22-point surgical global rating tool containing 6 subscales: depth of myometrial incision, ease of myoma-myometrium cleavage plane identification, ease of myoma detachment, blood loss during myoma detachment, myometrial blood loss after myoma detachment, and myoma consistency. MEASUREMENTS AND MAIN RESULTS: Participating surgeons submitted 55 videos of laparoscopic/robotic myomectomy procedures recorded over a 3-year period (2012-2015). Fifty met the inclusion criteria (25 UPA-treated patients and 25 patients without hormonal pretreatment). Patients treated with UPA were more likely to be older than patients with no medical pretreatment (mean age = 33.5 vs 38.3 years, p = .002). There were no statistically significant differences regarding other baseline characteristics such as the largest diameter of myoma removed, the number of myomas removed, or the estimated blood loss. There was no difference in the physician assessors' mean global rating score for patients with UPA pretreatment versus no pretreatment (12.4 vs 13.4, p = .23). Within the 6 subscales, no differences were observed between the 2 groups. Each video was graded independently by 2 assessors, and there was high inter-rater agreement for the total score and each subscale. CONCLUSION: There was no difference in surgical experience for myomectomies of patients pretreated with UPA versus those without medical pretreatment.


