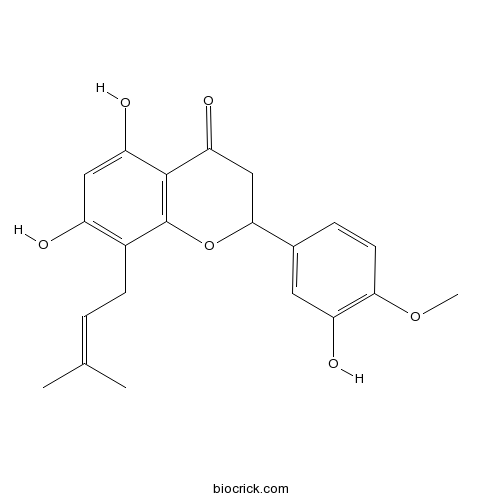
5,7,3'-Trihydroxy-4'-methoxy-8-prenylflavanoneCAS# 1268140-15-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1268140-15-3 | SDF | Download SDF |
| PubChem ID | 14259000 | Appearance | Powder |
| Formula | C21H22O6 | M.Wt | 370.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-8-(3-methylbut-2-enyl)-2,3-dihydrochromen-4-one | ||
| SMILES | CC(=CCC1=C(C=C(C2=C1OC(CC2=O)C3=CC(=C(C=C3)OC)O)O)O)C | ||
| Standard InChIKey | MDRKJMLXLVCUIU-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

5,7,3'-Trihydroxy-4'-methoxy-8-prenylflavanone Dilution Calculator

5,7,3'-Trihydroxy-4'-methoxy-8-prenylflavanone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6998 mL | 13.4989 mL | 26.9978 mL | 53.9957 mL | 67.4946 mL |
| 5 mM | 0.54 mL | 2.6998 mL | 5.3996 mL | 10.7991 mL | 13.4989 mL |
| 10 mM | 0.27 mL | 1.3499 mL | 2.6998 mL | 5.3996 mL | 6.7495 mL |
| 50 mM | 0.054 mL | 0.27 mL | 0.54 mL | 1.0799 mL | 1.3499 mL |
| 100 mM | 0.027 mL | 0.135 mL | 0.27 mL | 0.54 mL | 0.6749 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ulipristal acetate
Catalog No.:BCC4068
CAS No.:126784-99-4
- Sventenic acid
Catalog No.:BCN3923
CAS No.:126778-79-8
- GR 89696 fumarate
Catalog No.:BCC7083
CAS No.:126766-32-3
- Sarafotoxin S6a
Catalog No.:BCC5834
CAS No.:126738-34-9
- Acetylsventenic acid
Catalog No.:BCN4849
CAS No.:126737-42-6
- Tilifodiolide
Catalog No.:BCN6145
CAS No.:126724-95-6
- Gancaonin I
Catalog No.:BCN7144
CAS No.:126716-36-7
- Gancaonin G
Catalog No.:BCN6837
CAS No.:126716-34-5
- Isoaltenuene
Catalog No.:BCN7313
CAS No.:126671-80-5
- UTPγS trisodium salt
Catalog No.:BCC7624
CAS No.:1266569-94-1
- KT 5823
Catalog No.:BCC7006
CAS No.:126643-37-6
- 16-Epinormacusine B
Catalog No.:BCN4030
CAS No.:126640-98-0
- 13-Acetoxy-3beta-hydroxygermacra-1(10)E,4E,7(11)-trien-12,6alpha-olide
Catalog No.:BCN7314
CAS No.:126829-66-1
- 8alpha-Acetoxyarglabin
Catalog No.:BCN7315
CAS No.:126829-70-7
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- (-)-JQ1
Catalog No.:BCC3603
CAS No.:1268524-71-5
- MC 1046
Catalog No.:BCC1733
CAS No.:126860-83-1
- Ssioriside
Catalog No.:BCN6146
CAS No.:126882-53-9
- 3-Methoxy-5-heneicosylphenol
Catalog No.:BCN6147
CAS No.:126882-76-6
- MK 1903
Catalog No.:BCC6242
CAS No.:1268882-43-4
- LGX818
Catalog No.:BCC4184
CAS No.:1269440-17-6
- Sar-[D-Phe8]-des-Arg9-Bradykinin
Catalog No.:BCC5996
CAS No.:126959-88-4
- Methylenedihydrotanshinquinone
Catalog No.:BCN3221
CAS No.:126979-81-5
- Tetrahydro tanshinone I
Catalog No.:BCN2602
CAS No.:126979-84-8
Synthesis, Cytotoxic and Anti-proliferative Activity of Novel Thiophene, Thieno[2,3-b]pyridine and Pyran Derivatives Derived from 4,5,6,7-tetrahydrobenzo[b]thiophene Derivative.[Pubmed:28380235]
Acta Chim Slov. 2017 Mac;64(1):117-128.
Novel tetrahydrobenzo[b]thienopyrole derivatives are synthesized from 2-amino-3-cyano-4,5,6,7-tetrahydrobenzo[b]thiophene (1) through its reaction with alpha-chloroacetone to give the corresponding N-alkyl derivative 3. Compound 3 undergoes ready cyclization in sodium ethoxide solution to give the tetrahydrobenzo[b]thienopyrrole 4. The latter compound 4 is used as the key starting material for the synthesis of thiophene, thieno[2,3-b]pyridine and pyran derivatives. The cytotoxicity of the synthesized products towards the human cancer cell lines namely gastric cancer (NUGC), colon cancer (DLD-1), liver cancer (HA22T and HEPG-2), breast cancer (MCF-7), nasopharyngeal carcinoma (HONE-1) and normal fibroblast (WI-38) cell lines are measured. Compounds 4, 7a, 7b, 8a, 8b, 10c, 10d, 10f, 12a, 12b, 14b and 15b exhibit the optimal cytotoxic effect against cancer cell lines. Compounds 7b and 14b show the maximum inhibitory effect and these are much higher than the reference CHS-828 (pyridyl cyanoguanidine). On the other hand, the anti-proliferative evaluations of these compounds with high potency against the cancer cell lines L1210, Molt4/C8, CEM, K562, K562/4 and HCT116 show that compounds 7b and 8b give IC50's against Molt4/C8 and CEM cell lines higher than that of the reference, doxorubicin.
Colony-stimulating factor 2 acts from days 5 to 7 of development to modify programming of the bovine conceptus at day 86 of gestationdagger.[Pubmed:28379294]
Biol Reprod. 2017 Apr 1;96(4):743-757.
Colony-stimulating factor 2 (CSF2) is an embryokine that improves competence of the embryo to establish pregnancy and which may participate in developmental programming. We tested whether culture of bovine embryos with CSF2 alters fetal development and alleviates abnormalities associated with in vitro production (IVP) of embryos. Pregnancies were established by artificial insemination (AI), transfer of an IVP embryo (IVP), or transfer of an IVP embryo treated with 10 ng/ml CSF2 from day 5 to 7 of development (CSF2). Pregnancies were produced using X-sorted semen. Female singleton conceptuses were collected on day 86 of gestation. There were few morphological differences between groups, although IVP and CSF2 fetuses were heavier than AI fetuses. Bicarbonate concentration in allantoic fluid was lower for IVP than for AI or CSF2. Expression of 92 genes in liver, placenta, and muscle was determined. The general pattern for liver and placenta was for IVP to alter expression and for CSF2 to sometimes reverse this effect. For muscle, CSF2 affected gene expression but did not generally reverse effects of IVP. Levels of methylation for each of the three tissues at 12 loci in the promoter of insulin-like growth factor 2 (IGF2) and five in the promoter of growth factor receptor bound protein 10 were unaffected by treatment except for CSF2 effects on two CpG for IGF2 in placenta and muscle. In conclusion, CSF2 can act as a developmental programming agent but alone is not able to abolish the adverse effects of IVP on fetal characteristics.
Growth performance of immunologically castrated pigs slaughtered at 5, 7, or 9 weeks after the second Improvest dose and fed diets containing corn dried distillers grains with solubles.[Pubmed:28380585]
J Anim Sci. 2017 Feb;95(2):806-819.
Growth performance of immunologically castrated (IC) pigs (863 total) was determined at increasing time intervals between the second Improvest (gonadotropin releasing factor analog-diphtheria toxoid conjugate; Zoetis Inc., Florham Park, NJ) dose and slaughter (TD) and with 4 different dried distillers grains with solubles (DDGS) feeding strategies (FS) in a 4 x 3 factorial arrangement of treatments. The feeding period was divided into 4 separate diet phases. Dietary treatments included 1) corn-soybean meal control diets (PCon), 2) a gradual decrease of dietary DDGS inclusion rate from 40%, 30%, 20%, and 10% in phases 1 to 4 (GD), respectively, 3) feeding 40% DDGS diets in phases 1 to 3 and removal of DDGS from the phase 4 diet (WD), and 4) feeding 40% DDGS diets in all 4 phases (NCon). Pigs received the second Improvest dose at 9 (TD9), 7 (TD7), or 5 (TD5) wk before slaughter. In each group, all pigs were slaughtered on the same day. There were no 3-way interactions among FS, TD, and week of feeding period for any measure of growth performance. Pigs fed PCon and WD had greater ( < 0.05) overall ADFI than pigs fed NCon, especially when slaughtered 9 wk after the second Improvest dose (2.45 and 2.44 vs. 2.31 +/- 0.08 kg/d, respectively). This response was partly due to withdrawing DDGS from the diet at 19 wk of age (WD), which led to a tendency ( < 0.10) for increased ADFI from the wk 19 to 21 interval to the wk 21 to 24 interval (3.26 vs. 3.51 +/- 0.09 kg/d, respectively). During the same time period, ADFI was unchanged ( > 0.05) in pigs fed PCon, GD, and NCon. Overall G:F was improved ( < 0.05) in TD5 pigs compared with TD9 pigs and tended ( < 0.10) to be improved compared with TD7 pigs. Final BW was similar among pigs fed GD, WD, and PCon (123.1, 122.3, and 125.3 kg, respectively), but pigs fed PCon and GD had greater ( < 0.05) BW than pigs fed NCon (120.0 kg). Throughout the growing-finishing period, BW was similar among TD treatments. The GD FS was more effective than the WD FS in maintaining overall G:F (0.424 and 0.414 +/- 0.005, respectively) and ADG (0.94 and 0.93 +/- 0.03 kg/d, respectively), which were similar ( > 0.05) to those of pigs fed PCon (0.427 +/- 0.005 and 0.96 +/- 0.03 kg/d, respectively). Growth performance of pigs fed GD more closely reflected that of pigs fed PCon than that of pigs fed WD. Delaying the second dose of Improvest from 9 to 5 wk before slaughter resulted in improved growth performance.
Investigation of Biodistribution and Speciation Changes of Orally Administered Dual Radiolabeled Complex, Bis(5-chloro-7-[(131)I]iodo-8-quinolinolato)[(65)Zn]zinc.[Pubmed:28381805]
Biol Pharm Bull. 2017;40(4):510-515.
Many zinc (Zn) complexes have been developed as promising oral antidiabetic agents. In vitro assays using adipocytes have demonstrated that the coordination structures of Zn complexes affect the uptake of Zn into cells and have insulinomimetic activities, for which moderate stability of Zn complexes is vital. The complexation of Zn plays a major role improving its bioavailability. However, investigation of the speciation changes of Zn complexes after oral administration is lacking. A dual radiolabeling approach was applied in order to investigate the speciation of bis(5-chloro-7-iodo-8-quinolinolato)zinc complex [Zn(Cq)2], which exhibits the antidiabetic activity in diabetic mice. In the present study, (65)Zn- and (131)I-labeled [Zn(Cq)2] were synthesized, and their biodistribution were analyzed after an oral administration using both invasive conventional assays and noninvasive gamma-ray emission imaging (GREI), a novel nuclear medicine imaging modality that enables analysis of multiple radionuclides simultaneously. The GREI experiments visualized the behavior of (65)Zn and [(131)I]Cq from the stomach to large intestine and through the small intestine; most of the administered Zn was transported together with clioquinol (5-chloro-7-iodo-8-quinolinol) (Cq). Higher accumulation of (65)Zn for [Zn(Cq)2] than ZnCl2 suggests that the Zn associated with Cq was highly absorbed by the intestinal tract. In particular, the molar ratio of administered iodine to Zn decreased during the distribution processes, indicating the dissociation of most [Zn(Cq)2] complexes. In conclusion, the present study successfully evaluated the speciation changes of orally administered [Zn(Cq)2] using the dual radiolabeling method.


