GR 89696 fumarateCAS# 126766-32-3 |
- GPR120 modulator 1
Catalog No.:BCC1599
CAS No.:1050506-75-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 126766-32-3 | SDF | Download SDF |
| PubChem ID | 6442840 | Appearance | Powder |
| Formula | C23H29Cl2N3O7 | M.Wt | 530.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in water with gentle warming and to 100 mM in DMSO | ||
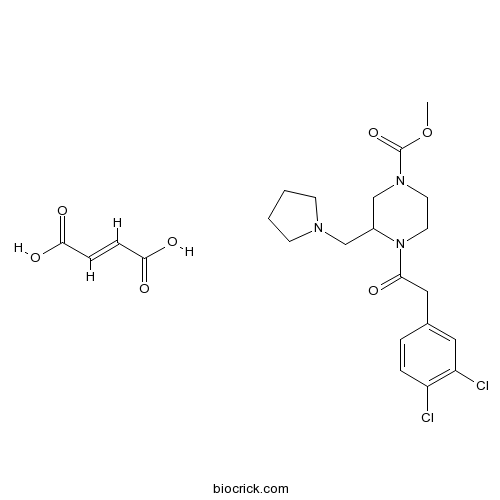
| Chemical Name | (E)-but-2-enedioic acid;methyl 4-[2-(3,4-dichlorophenyl)acetyl]-3-(pyrrolidin-1-ylmethyl)piperazine-1-carboxylate | ||
| SMILES | COC(=O)N1CCN(C(C1)CN2CCCC2)C(=O)CC3=CC(=C(C=C3)Cl)Cl.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | ABTNETSDXZBJTE-WLHGVMLRSA-N | ||
| Standard InChI | InChI=1S/C19H25Cl2N3O3.C4H4O4/c1-27-19(26)23-8-9-24(15(13-23)12-22-6-2-3-7-22)18(25)11-14-4-5-16(20)17(21)10-14;5-3(6)1-2-4(7)8/h4-5,10,15H,2-3,6-9,11-13H2,1H3;1-2H,(H,5,6)(H,7,8)/b;2-1+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly potent and selective κ-opioid agonist (IC50 = 0.04 nM) that may be selective for the putative κ2 receptor. Anti-nociceptive and neuroprotective in vivo. |

GR 89696 fumarate Dilution Calculator

GR 89696 fumarate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8854 mL | 9.4268 mL | 18.8537 mL | 37.7074 mL | 47.1342 mL |
| 5 mM | 0.3771 mL | 1.8854 mL | 3.7707 mL | 7.5415 mL | 9.4268 mL |
| 10 mM | 0.1885 mL | 0.9427 mL | 1.8854 mL | 3.7707 mL | 4.7134 mL |
| 50 mM | 0.0377 mL | 0.1885 mL | 0.3771 mL | 0.7541 mL | 0.9427 mL |
| 100 mM | 0.0189 mL | 0.0943 mL | 0.1885 mL | 0.3771 mL | 0.4713 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sarafotoxin S6a
Catalog No.:BCC5834
CAS No.:126738-34-9
- Acetylsventenic acid
Catalog No.:BCN4849
CAS No.:126737-42-6
- Tilifodiolide
Catalog No.:BCN6145
CAS No.:126724-95-6
- Gancaonin I
Catalog No.:BCN7144
CAS No.:126716-36-7
- Gancaonin G
Catalog No.:BCN6837
CAS No.:126716-34-5
- Isoaltenuene
Catalog No.:BCN7313
CAS No.:126671-80-5
- UTPγS trisodium salt
Catalog No.:BCC7624
CAS No.:1266569-94-1
- KT 5823
Catalog No.:BCC7006
CAS No.:126643-37-6
- 16-Epinormacusine B
Catalog No.:BCN4030
CAS No.:126640-98-0
- A 887826
Catalog No.:BCC7898
CAS No.:1266212-81-0
- Trilobinine
Catalog No.:BCN7927
CAS No.:126595-92-4
- Vallesamine N-oxide
Catalog No.:BCN6144
CAS No.:126594-73-8
- Sventenic acid
Catalog No.:BCN3923
CAS No.:126778-79-8
- Ulipristal acetate
Catalog No.:BCC4068
CAS No.:126784-99-4
- 5,7,3'-Trihydroxy-4'-methoxy-8-prenylflavanone
Catalog No.:BCN1590
CAS No.:1268140-15-3
- 13-Acetoxy-3beta-hydroxygermacra-1(10)E,4E,7(11)-trien-12,6alpha-olide
Catalog No.:BCN7314
CAS No.:126829-66-1
- 8alpha-Acetoxyarglabin
Catalog No.:BCN7315
CAS No.:126829-70-7
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- (-)-JQ1
Catalog No.:BCC3603
CAS No.:1268524-71-5
- MC 1046
Catalog No.:BCC1733
CAS No.:126860-83-1
- Ssioriside
Catalog No.:BCN6146
CAS No.:126882-53-9
- 3-Methoxy-5-heneicosylphenol
Catalog No.:BCN6147
CAS No.:126882-76-6
- MK 1903
Catalog No.:BCC6242
CAS No.:1268882-43-4
- LGX818
Catalog No.:BCC4184
CAS No.:1269440-17-6
GR89,696: a potent kappa-opioid agonist with subtype selectivity in rhesus monkeys.[Pubmed:11504802]
J Pharmacol Exp Ther. 2001 Sep;298(3):1049-59.
GR89,696 is a synthetic kappa-opioid receptor agonist, recently reported to have an agonist profile consistent with selectivity at the proposed "kappa(2)" subtype. The present studies evaluated the effects of GR89,696 in vitro (i.e., in radioligand binding and [(35)S]guanosine-5'-O-(3-thio)triphosphate assays) and in vivo in rhesus monkeys, in assays used to study kappa-opioid agonists (i.e., thermal antinociception, sedation and muscle relaxation, diuresis, and increases in serum prolactin levels, as well as ethylketocyclazocine and U69,593 discrimination). Furthermore, the sensitivity of GR89,696 to naltrexone and nor-binaltorphimine (nor-BNI) antagonism was compared with that of U50,488 and U69,593, ligands selective for the proposed "kappa(1)" subtype. Overall, GR89,696 displayed the profile of a highly potent kappa-opioid agonist, following parenteral administration in rhesus monkeys. GR89,696 was less sensitive than U50,488 and U69,593 to naltrexone or nor-BNI antagonism, consistent with an action through the proposed kappa(2) receptor subtype.
A potent new class of kappa-receptor agonist: 4-substituted 1-(arylacetyl)-2-[(dialkylamino)methyl]piperazines.[Pubmed:8393489]
J Med Chem. 1993 Jul 23;36(15):2075-83.
The synthesis of 4-substituted 1-(arylacetyl)-2-[(alkylamino)methyl]piperazines (10-22, 26, 27, and 30-33) and their activities as kappa-opioid receptor agonists are described. This includes a range of 4-acyl and 4-carboalkoxy derivatives with the latter series showing the greatest kappa-agonist activity. In particular, methyl 4-[(3,4-dichlorophenyl)acetyl]-3-[(1-pyrrolidinyl) methyl]-1-piperazinecarboxylate (18) displays exceptional potency and selectivity. It showed the following activities in functional in vitro assays: rabbit vas deferens (kappa-specific tissue) IC50 = 0.041 nM, rat vas deferens (mu-specific tissue) IC50 > 10,000 nM, and hamster vas deferens (delta-specific tissue) IC50 > 10,000 nM. Compound 18 is also a highly potent antinociceptive agent, as determined in the mouse acetylcholine-induced abdominal constriction test: ED50 = 0.000 52 mg/kg, sc. The activity of 18 resides solely in its 3(R)-enantiomer. The kappa-agonist activity in both the 4-acyl and the 4-carbamate series is sensitive to the size of the 4-substituent. In addition, it would appear that an appreciable negative electrostatic potential in this region of the molecule is an important requirement for optimal potency.
Neuroprotective actions of GR89696, a highly potent and selective kappa-opioid receptor agonist.[Pubmed:1657267]
Br J Pharmacol. 1991 Jul;103(3):1819-23.
1. The effect of a novel, highly potent and selective kappa-opioid receptor agonist, GR89696, has been evaluated in two animal models of cerebral ischaemia: transient bilateral carotid artery occlusion in the Mongolian gerbil and permanent, unilateral middle cerebral artery occlusion in the mouse. 2. In the Mongolian gerbil model, administration of GR89696 (3 to 30 micrograms kg-1, s.c.), immediately before and at 4 h after insult, produced a dose-dependent reduction in the hippocampal CA1 neuronal cell loss resulting from a 7-min bilateral carotid occlusion. Similar effects were obtained with two other kappa-agonists, GR86014 (1 mgkg-1, s.c.) and GR91272 (1 mgkg-1, s.c.). The neuroprotective effect of GR89696 was completely blocked by prior administration of the opioid receptor antagonist, naltrexone, at 10 mgkg-1, s.c. Repeated post-treatment with GR89696 (100 micrograms kg-1, s.c.) or GR44821 (10 mgkg-1, s.c.) was also effective in protecting completely the hippocampal CA1 neurones from ischaemia-induced neurodegeneration. 3. In the permanent, unilateral middle cerebral artery occlusion model in the mouse, repeated administration of GR89696 at 300 micrograms kg-1, s.c. produced a 50% reduction in cerebrocortical infarct volume. In these experiments GR89696 was dosed 5 min, 4, 8, 12, 16, 20 and 24 h after occlusion on the first day and then three times daily for the next three days. GR89696 (300 micrograms kg-1) also produced a significant 35% reduction in infarct volume in this model when the initiation of dosing was delayed for 6 h after the insult. 4. The results indicate that the potent kappa-opioid receptor agonist, GR89696, is neuroprotective in both global and focal cerebral ischaemia models and suggest that, with this class of compound, there may be a considerable time window for pharmacological intervention.


