AR-C155858MCT1 and MCT2 inhibitor CAS# 496791-37-8 |
- UK-5099
Catalog No.:BCC2021
CAS No.:56396-35-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 496791-37-8 | SDF | Download SDF |
| PubChem ID | 10226546 | Appearance | Powder |
| Formula | C21H27N5O5S | M.Wt | 461.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (108.34 mM) *"≥" means soluble, but saturation unknown. | ||
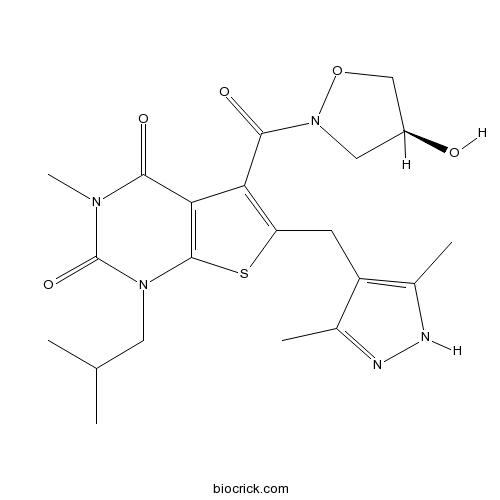
| Chemical Name | 6-[(3,5-dimethyl-1H-pyrazol-4-yl)methyl]-5-[(4S)-4-hydroxy-1,2-oxazolidine-2-carbonyl]-3-methyl-1-(2-methylpropyl)thieno[2,3-d]pyrimidine-2,4-dione | ||
| SMILES | CC1=C(C(=NN1)C)CC2=C(C3=C(S2)N(C(=O)N(C3=O)C)CC(C)C)C(=O)N4CC(CO4)O | ||
| Standard InChIKey | ISIVOJWVBJIOFM-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C21H27N5O5S/c1-10(2)7-25-20-17(18(28)24(5)21(25)30)16(19(29)26-8-13(27)9-31-26)15(32-20)6-14-11(3)22-23-12(14)4/h10,13,27H,6-9H2,1-5H3,(H,22,23)/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of the monocarboxylate transporters (MCTs) MCT1 and MCT2 (Ki values are 2.3 and <10 nM respectively). Exhibits no activity at MCT4. Blocks proliferation of Raji lymphoma cells in vitro. Inhibits glycolysis and glutathione synthesis in cancer cells. |

AR-C155858 Dilution Calculator

AR-C155858 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1667 mL | 10.8335 mL | 21.6671 mL | 43.3341 mL | 54.1677 mL |
| 5 mM | 0.4333 mL | 2.1667 mL | 4.3334 mL | 8.6668 mL | 10.8335 mL |
| 10 mM | 0.2167 mL | 1.0834 mL | 2.1667 mL | 4.3334 mL | 5.4168 mL |
| 50 mM | 0.0433 mL | 0.2167 mL | 0.4333 mL | 0.8667 mL | 1.0834 mL |
| 100 mM | 0.0217 mL | 0.1083 mL | 0.2167 mL | 0.4333 mL | 0.5417 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AR-C155858 is a potent and selective inhibitor of monocarboxylate transporters MCT1 and MCT2 with Ki values of 2.3nM and less than 10 nM, respectively [1].
MCT is a monocarboxylate transporter family with 14 members. Among these transporters, MCT1 and MCT2 are worked for the uptake and efflux of lactic acid. As a selective inhibitor of MCT1 and MCT2, AR-C155858 can be used to probe MCTs’ roles in the metabolic studies. Besides that, AR-C155858 was found to have immunosuppressive activity and can inhibit the proliferation of T-lymphocytes. The binding site of MCT1 for AR-C155858 contains transmembrane helices 7-10 in C-terminal domain. The two residues Phe360 and Ser364 play important roles in the binding [1 and 2].
In rat erythrocytes in which the AE1 mediated lactate transport had been blocked, AR-C155858 inhibited endogenous MCT1 mediated L-lactate uptake dose-dependently. AR-C155858 showed to be a tight-binding non-competitive inhibitor with Ki value of 2.3±1.4 nM and Kcat value of 12.2±1.1 s-1.In Xenopus oocytes expressing MCT1, MCT2 or MCT4, AR-C155858 at concentration of 100 nM showed 100% inhibition of MCT1 mediated lactate uptake. For MCT2, AR-C155858 at 10 nM showed 70% inhibition. AR-C155858 had no significant effect against MCT4 even at concentration up to 10 μM. In addition, it has been found that the inhibition of MCTs caused by AR-C155858 can be affected by the co-expression of ancillary proteins. The presence of embigin reduced the sensitivity of MCT2 against AR-C155858’s inhibition. AR-C155858 potently suppressed the uptake of lactic acid in Ras-transformed fibroblast CCL39 cells via inhibiting MCT1 and MCT2 but no MCT4. The suppression subsequently resulted in significant decrease of glycolysis [1, 3 and 4].
In nude mice implanted with Ras-transformed CCL39 fibroblasts, the administration of AR-C155858 at dose of 30 mg/kg twice daily resulted in significant tumor growth suppression [4].
References:
[1] Ovens MJ, Davies AJ, Wilson MC, Murray CM, Halestrap AP. AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7-10. Biochem J. 2010 Jan 15;425(3):523-30.
[2] Nancolas B, Sessions RB, Halestrap AP. Identification of key binding site residues of MCT1 for AR-C155858 reveals the molecular basis of its isoform selectivity. Biochem J. 2015 Feb 15;466(1):177-88.
[3] Ovens MJ, Manoharan C, Wilson MC, Murray CM, Halestrap AP. The inhibition of monocarboxylate transporter 2 (MCT2) by AR-C155858 is modulated by the associated ancillary protein. Biochem J. 2010 Oct 15;431(2):217-25.
[4] Le Floch R, Chiche J, Marchiq I, Naiken T, Ilc K, Murray CM, Critchlow SE, Roux D, Simon MP, Pouysségur J. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc Natl Acad Sci U S A. 2011 Oct 4;108(40):16663-8.
- Crobarbatine
Catalog No.:BCN2069
CAS No.:49679-23-4
- Eltrombopag Olamine
Catalog No.:BCC1549
CAS No.:496775-62-3
- Eltrombopag
Catalog No.:BCC4968
CAS No.:496775-61-2
- ZLN005
Catalog No.:BCC4882
CAS No.:49671-76-3
- Simiarenol acetate
Catalog No.:BCN5606
CAS No.:4965-99-5
- Isomitraphylline
Catalog No.:BCN7800
CAS No.:4963-01-3
- Angelicain
Catalog No.:BCN5605
CAS No.:49624-66-0
- Robustaflavone
Catalog No.:BCN8285
CAS No.:49620-13-5
- Tetraethylenepentamine 5HCl
Catalog No.:BCC3867
CAS No.:4961-41-5
- Helicianeoide B
Catalog No.:BCN2487
CAS No.:496066-89-8
- Helicianeoide A
Catalog No.:BCN2486
CAS No.:496066-82-1
- Pyromeconic acid
Catalog No.:BCN7177
CAS No.:496-63-9
- HhAntag
Catalog No.:BCC1617
CAS No.:496794-70-8
- Drupacine
Catalog No.:BCN7065
CAS No.:49686-57-9
- Adarotene
Catalog No.:BCC1328
CAS No.:496868-77-0
- Arbutin
Catalog No.:BCN6307
CAS No.:497-76-7
- (-)-Praeruptorin B
Catalog No.:BCN7665
CAS No.:4970-26-7
- Z-Thr-NH2
Catalog No.:BCC2738
CAS No.:49705-98-8
- DC_AC50
Catalog No.:BCC6488
CAS No.:497061-48-0
- Tupichinol A
Catalog No.:BCN7697
CAS No.:497142-88-8
- Dobutamine hydrochloride
Catalog No.:BCC5391
CAS No.:49745-95-1
- H-D-Phe(4-Me)-OH
Catalog No.:BCC3271
CAS No.:49759-61-7
- Stiripentol
Catalog No.:BCC3977
CAS No.:49763-96-4
- 11beta-Hydroxylupeol
Catalog No.:BCN7571
CAS No.:49776-92-3
The inhibition of monocarboxylate transporter 2 (MCT2) by AR-C155858 is modulated by the associated ancillary protein.[Pubmed:20695846]
Biochem J. 2010 Oct 15;431(2):217-25.
In mammalian cells, MCTs (monocarboxylate transporters) require association with an ancillary protein to enable plasma membrane expression of the active transporter. Basigin is the preferred binding partner for MCT1, MCT3 and MCT4, and embigin for MCT2. In rat and rabbit erythrocytes, MCT1 is associated with embigin and basigin respectively, but its sensitivity to inhibition by AR-C155858 was found to be identical. Using RT (reverse transcription)-PCR, we have shown that Xenopus laevis oocytes contain endogenous basigin, but not embigin. Co-expression of exogenous embigin was without effect on either the expression of MCT1 or its inhibition by AR-C155858. In contrast, expression of active MCT2 at the plasma membrane of oocytes was significantly enhanced by co-expression of exogenous embigin. This additional transport activity was insensitive to inhibition by AR-C155858 unlike that by MCT2 expressed with endogenous basigin that was potently inhibited by AR-C155858. Chimaeras and C-terminal truncations of MCT1 and MCT2 were also expressed in oocytes in the presence and absence of exogenous embigin. L-Lactate Km values for these constructs were determined and revealed that the TM (transmembrane) domains of an MCT, most probably TM7-TM12, but not the C-terminus, are the major determinants of L-lactate affinity, whereas the associated ancillary protein has little or no effect. Inhibitor titrations of lactate transport by these constructs indicated that embigin modulates MCT2 sensitivity to AR-C155858 through interactions with both the intracellular C-terminus and TMs 3 and 6 of MCT2. The C-terminus of MCT2 was found to be essential for its expression with endogenous basigin.
AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7-10.[Pubmed:19929853]
Biochem J. 2010 Jan 15;425(3):523-30.
In the present study we characterize the properties of the potent MCT1 (monocarboxylate transporter 1) inhibitor AR-C155858. Inhibitor titrations of L-lactate transport by MCT1 in rat erythrocytes were used to determine the Ki value and number of AR-C155858-binding sites (Et) on MCT1 and the turnover number of the transporter (kcat). Derived values were 2.3+/-1.4 nM, 1.29+/-0.09 nmol per ml of packed cells and 12.2+/-1.1 s-1 respectively. When expressed in Xenopus laevis oocytes, MCT1 and MCT2 were potently inhibited by AR-C155858, whereas MCT4 was not. Inhibition of MCT1 was shown to be time-dependent, and the compound was also active when microinjected, suggesting that AR-C155858 probably enters the cell before binding to an intracellular site on MCT1. Measurement of the inhibitor sensitivity of several chimaeric transporters combining different domains of MCT1 and MCT4 revealed that the binding site for AR-C155858 is contained within the C-terminal half of MCT1, and involves TM (transmembrane) domains 7-10. This is consistent with previous data identifying Phe360 (in TM10) and Asp302 plus Arg306 (TM8) as key residues in substrate binding and translocation by MCT1. Measurement of the Km values of the chimaeras for L-lactate and pyruvate demonstrate that both the C- and N-terminal halves of the molecule influence transport kinetics consistent with our proposed molecular model of MCT1 and its translocation mechanism that requires Lys38 in TM1 in addition to Asp302 and Arg306 in TM8 [Wilson, Meredith, Bunnun, Sessions and Halestrap (2009) J. Biol. Chem. 284, 20011-20021].
Identification of key binding site residues of MCT1 for AR-C155858 reveals the molecular basis of its isoform selectivity.[Pubmed:25437897]
Biochem J. 2015 Feb 15;466(1):177-88.
The proton-linked monocarboxylate transporters (MCTs) are required for lactic acid transport into and out of all mammalian cells. Thus, they play an essential role in tumour cells that are usually highly glycolytic and are promising targets for anti-cancer drugs. AR-C155858 is a potent MCT1 inhibitor (Ki ~2 nM) that also inhibits MCT2 when associated with basigin but not MCT4. Previous work [Ovens, M.J. et al. (2010) Biochem. J. 425, 523-530] revealed that AR-C155858 binding to MCT1 occurs from the intracellular side and involves transmembrane helices (TMs) 7-10. In the present paper, we generate a molecular model of MCT4 based on our previous models of MCT1 and identify residues in the intracellular substrate-binding cavity that differ significantly between MCT4 and MCT1/MCT2 and so might account for differences in inhibitor binding. We tested their involvement using site-directed mutagenesis (SDM) of MCT1 to change residues individually or in combination with their MCT4 equivalent and determined inhibitor sensitivity following expression in Xenopus oocytes. Phe360 and Ser364 were identified as important for AR-C155858 binding with the F360Y/S364G mutant exhibiting >100-fold reduction in inhibitor sensitivity. To refine the binding site further, we used molecular dynamics (MD) simulations and additional SDM. This approach implicated six more residues whose involvement was confirmed by both transport studies and [3H]-AR-C155858 binding to oocyte membranes. Taken together, our data imply that Asn147, Arg306 and Ser364 are important for directing AR-C155858 to its final binding site which involves interaction of the inhibitor with Lys38, Asp302 and Phe360 (residues that also play key roles in the translocation cycle) and also Leu274 and Ser278.
Blocking lactate export by inhibiting the Myc target MCT1 Disables glycolysis and glutathione synthesis.[Pubmed:24285728]
Cancer Res. 2014 Feb 1;74(3):908-20.
Myc oncoproteins induce genes driving aerobic glycolysis, including lactate dehydrogenase-A that generates lactate. Here, we report that Myc controls transcription of the lactate transporter SLC16A1/MCT1 and that elevated MCT1 levels are manifest in premalignant and neoplastic Emu-Myc transgenic B cells and in human malignancies with MYC or MYCN involvement. Notably, disrupting MCT1 function leads to an accumulation of intracellular lactate that rapidly disables tumor cell growth and glycolysis, provoking marked alterations in glycolytic intermediates, reductions in glucose transport, and in levels of ATP, NADPH, and ultimately, glutathione (GSH). Reductions in GSH then lead to increases in hydrogen peroxide, mitochondrial damage, and ultimately, cell death. Finally, forcing glycolysis by metformin treatment augments this response and the efficacy of MCT1 inhibitors, suggesting an attractive combination therapy for MYC/MCT1-expressing malignancies.


