UK-5099MCTs and MPC inhibitor CAS# 56396-35-1 |
- AR-C155858
Catalog No.:BCC1367
CAS No.:496791-37-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 56396-35-1 | SDF | Download SDF |
| PubChem ID | 6438504 | Appearance | Powder |
| Formula | C18H12N2O2 | M.Wt | 288.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | PF-1005023 | ||
| Solubility | DMSO : ≥ 50 mg/mL (173.43 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
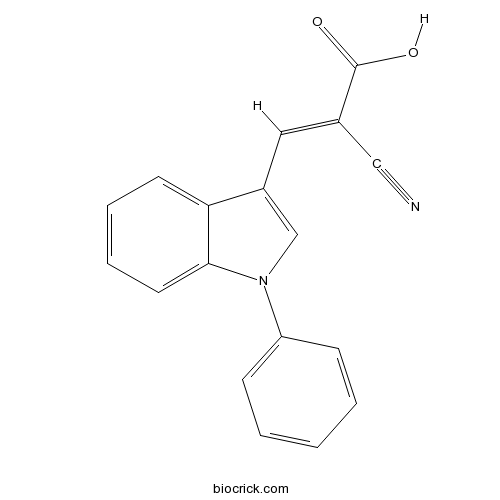
| Chemical Name | (E)-2-cyano-3-(1-phenylindol-3-yl)prop-2-enoic acid | ||
| SMILES | C1=CC=C(C=C1)N2C=C(C3=CC=CC=C32)C=C(C#N)C(=O)O | ||
| Standard InChIKey | BIZNHCWFGNKBBZ-JLHYYAGUSA-N | ||
| Standard InChI | InChI=1S/C18H12N2O2/c19-11-13(18(21)22)10-14-12-20(15-6-2-1-3-7-15)17-9-5-4-8-16(14)17/h1-10,12H,(H,21,22)/b13-10+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of plasma membrane monocarboxylate transporters (MCTs) and the mitochondrial pyruvate carrier (MPC). |

UK-5099 Dilution Calculator

UK-5099 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4686 mL | 17.343 mL | 34.6861 mL | 69.3722 mL | 86.7152 mL |
| 5 mM | 0.6937 mL | 3.4686 mL | 6.9372 mL | 13.8744 mL | 17.343 mL |
| 10 mM | 0.3469 mL | 1.7343 mL | 3.4686 mL | 6.9372 mL | 8.6715 mL |
| 50 mM | 0.0694 mL | 0.3469 mL | 0.6937 mL | 1.3874 mL | 1.7343 mL |
| 100 mM | 0.0347 mL | 0.1734 mL | 0.3469 mL | 0.6937 mL | 0.8672 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
UK-5099 is a potent inhibitor of the mitochondrial pyruvate carrier [1].
The mitochondrial pyruvate carrier (MPC) facilitates pyruvate transport across the mitochondrial inner membrane and plays a critical role in carbohydrate, lipid and amino acid metabolism.
UK-5099 (1 mM) completely blocked pyruvate uptake with Ki value of 49 μM. Also, UK-5099 decreased the overall efflux rate in a concentration-dependant way [1]. In mitochondria isolated from S. Guttatum, UK-5099 (20 μM) inhibited pyruvate-dependent 02 consumption [2].
In rat heart mitochondria, UK-5099 inhibited pyruvate oxidation with a non-linear inhibition kinetics [2]. In glucagon-treated rats, UK-5099 inhibited pyruvate carboxylation and total pyruvate metabolism with a linear relationship [3]. In rat liver and heart mitochondria, UK-5099 inhibited pyruvate-dependent 02 consumption with IC50 value of 50 nM [4].
References:
[1]. Wiemer EA, Michels PA, Opperdoes FR. The inhibition of pyruvate transport across the plasma membrane of the bloodstream form of Trypanosoma brucei and its metabolic implications. Biochem J, 1995, 312 ( Pt 2): 479-484.
[2]. Halestrap AP. The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. Biochem J, 1975, 148(1): 85-96.
[3]. Halestrap AP, Armston AE. A re-evaluation of the role of mitochondrial pyruvate transport in the hormonal control of rat liver mitochondrial pyruvate metabolism. Biochem J, 1984, 223(3): 677-685.
[4]. Proudlove MO, Beechey RB, Moore AL. Pyruvate transport by thermogenic-tissue mitochondria. Biochem J, 1987, 247(2): 441-447.
- Metoprolol Tartrate
Catalog No.:BCC4330
CAS No.:56392-17-7
- Netilmicin Sulfate
Catalog No.:BCC4683
CAS No.:56391-57-2
- Epirubicin HCl
Catalog No.:BCC1192
CAS No.:56390-09-1
- Tirotundin
Catalog No.:BCN5754
CAS No.:56377-67-4
- Capillarisin
Catalog No.:BCN2461
CAS No.:56365-38-9
- Oxybutynin
Catalog No.:BCC3833
CAS No.:5633-20-5
- Hedychenone
Catalog No.:BCN5753
CAS No.:56324-54-0
- Conocarpan acetate
Catalog No.:BCN7584
CAS No.:56319-04-1
- Moracin M
Catalog No.:BCN3292
CAS No.:56317-21-6
- 5,6,7,8-Tetramethoxycoumarin
Catalog No.:BCN5752
CAS No.:56317-15-8
- Kaempferol 3-O-(6''-galloyl)-beta-D-glucopyranoside
Catalog No.:BCN1416
CAS No.:56317-05-6
- Trimethylapigenin
Catalog No.:BCN8081
CAS No.:5631-70-9
- Hop-17(21)-en-3-ol
Catalog No.:BCN5755
CAS No.:564-14-7
- Sclareolide
Catalog No.:BCC6492
CAS No.:564-20-5
- Doxycycline
Catalog No.:BCN2397
CAS No.:564-25-0
- 11α-Hydroxyandrost-4-ene-3,17-dione
Catalog No.:BCC8432
CAS No.:564-33-0
- Hinokiol
Catalog No.:BCN5759
CAS No.:564-73-8
- Tetrahydrolachnophyllum lactone
Catalog No.:BCN4759
CAS No.:56407-87-5
- Methyl eichlerianate
Catalog No.:BCN5756
CAS No.:56421-12-6
- Eichlerianic acid
Catalog No.:BCN5757
CAS No.:56421-13-7
- 4,9-Dihydroxy-alpha-lapachone
Catalog No.:BCN5758
CAS No.:56473-67-7
- Steppogenin
Catalog No.:BCN5760
CAS No.:56486-94-3
- Angelic acid
Catalog No.:BCN3410
CAS No.:565-63-9
- 2-Hydroxy-3,4-dimethoxybenzoic acid
Catalog No.:BCN6535
CAS No.:5653-46-3
Lactate dehydrogenase activity drives hair follicle stem cell activation.[Pubmed:28812580]
Nat Cell Biol. 2017 Sep;19(9):1017-1026.
Although normally dormant, hair follicle stem cells (HFSCs) quickly become activated to divide during a new hair cycle. The quiescence of HFSCs is known to be regulated by a number of intrinsic and extrinsic mechanisms. Here we provide several lines of evidence to demonstrate that HFSCs utilize glycolytic metabolism and produce significantly more lactate than other cells in the epidermis. Furthermore, lactate generation appears to be critical for the activation of HFSCs as deletion of lactate dehydrogenase (Ldha) prevented their activation. Conversely, genetically promoting lactate production in HFSCs through mitochondrial pyruvate carrier 1 (Mpc1) deletion accelerated their activation and the hair cycle. Finally, we identify small molecules that increase lactate production by stimulating Myc levels or inhibiting Mpc1 carrier activity and can topically induce the hair cycle. These data suggest that HFSCs maintain a metabolic state that allows them to remain dormant and yet quickly respond to appropriate proliferative stimuli.
Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism.[Pubmed:28812582]
Nat Cell Biol. 2017 Sep;19(9):1027-1036.
Most differentiated cells convert glucose to pyruvate in the cytosol through glycolysis, followed by pyruvate oxidation in the mitochondria. These processes are linked by the mitochondrial pyruvate carrier (MPC), which is required for efficient mitochondrial pyruvate uptake. In contrast, proliferative cells, including many cancer and stem cells, perform glycolysis robustly but limit fractional mitochondrial pyruvate oxidation. We sought to understand the role this transition from glycolysis to pyruvate oxidation plays in stem cell maintenance and differentiation. Loss of the MPC in Lgr5-EGFP-positive stem cells, or treatment of intestinal organoids with an MPC inhibitor, increases proliferation and expands the stem cell compartment. Similarly, genetic deletion of the MPC in Drosophila intestinal stem cells also increases proliferation, whereas MPC overexpression suppresses stem cell proliferation. These data demonstrate that limiting mitochondrial pyruvate metabolism is necessary and sufficient to maintain the proliferation of intestinal stem cells.
A mathematical model predicting host mitochondrial pyruvate transporter activity to be a critical regulator of Mycobacterium tuberculosis pathogenicity.[Pubmed:28263840]
Biosystems. 2017 May;155:1-9.
Modulation of host metabolic machinery by Mycobacterium tuberculosis is a well established phenomenon. In our earlier study (Mehrotra et al., 2014), we observed a marked increase in acetyl-CoA levels in cells bearing virulent M. tuberculosis infections compared to host cells harbouring avirulent infections. The difference was observed inspite of similar levels of total host cellular pyruvate in both infection types. The present study aimed in capturing the cause for such a phenomenon that defines the pathogenicity of M. tuberculosis. Through mathematical model, we dissected the relative importance of virulence mediated effect on Pyruvate dehydrogenase (PDH) activity, rate of acetyl-CoA consumption and mitochondrial pyruvate transporter (MPC) activity in causing the observed outcomes. Simulation results exhibit MPC to be the key regulatory junction perturbed by virulent strains of M. tuberculosis leading to alteration of mitochondrial metabolic flux and regulation of acetyl-CoA formation. As an experimental validation, drug mediated inhibition of MPC activity was sufficient to reduce virulent bacillary loads, pointing towards a possible mechanistic target for drug discovery.
Application of mitochondrial pyruvate carrier blocker UK5099 creates metabolic reprogram and greater stem-like properties in LnCap prostate cancer cells in vitro.[Pubmed:26413751]
Oncotarget. 2015 Nov 10;6(35):37758-69.
Aerobic glycolysis is one of the important hallmarks of cancer cells and eukaryotic cells. In this study, we have investigated the relationship between blocking mitochondrial pyruvate carrier (MPC) with UK5099 and the metabolic alteration as well as stemness phenotype of prostatic cancer cells. It was found that blocking pyruvate transportation into mitochondrial attenuated mitochondrial oxidative phosphorylation (OXPHOS) and increased glycolysis. The UK5099 treated cells showed significantly higher proportion of side population (SP) fraction and expressed higher levels of stemness markers Oct3/4 and Nanog. Chemosensitivity examinations revealed that the UK5099 treated cells became more resistant to chemotherapy compared to the non-treated cells. These results demonstrate probably an intimate connection between metabolic reprogram and stem-like phenotype of LnCap cells in vitro. We propose that MPC blocker (UK5099) application may be an ideal model for Warburg effect studies, since it attenuates mitochondrial OXPHOS and increases aerobic glycolysis, a phenomenon typically reflected in the Warburg effect. We conclude that impaired mitochondrial OXPHOS and upregulated glycolysis are related with stem-like phenotype shift in prostatic cancer cells.
Cinnamic Acid Derivatives Enhance the Efficacy of Transarterial Embolization in a Rat Model of Hepatocellular Carcinoma.[Pubmed:27872984]
Cardiovasc Intervent Radiol. 2017 Mar;40(3):430-437.
INTRODUCTION: We hypothesize that the combination of transarterial embolization (TAE) plus inhibition of lactate export will limit anaerobic metabolism and reduce tumor survival compared to TAE alone. The purpose of this study was to test this hypothesis in a rat model of hepatocellular carcinoma (HCC). METHODS: Rat N1-S1 hepatoma cells were assayed in vitro using the Seahorse XF analyzer to measure extracellular acidification (lactate excretion) comparing effects of the addition of caffeic acid (CA) or ferulic acid (FA) or UK-5099 with control. Monocarboxylate transporter Slc16a3 was knocked down by RNAi. N1S1 tumors were orthotopically implanted in rats and 4 groups evaluated: (1) Control, (2) TAE-only, (3) TAE plus CA, and (4) TAE plus FA. Tumor size was determined by ultrasound and analyzed by repeated measures statistics. Tumors harvested at 4 weeks were examined by microscopy. RESULTS: Seahorse assays showed that CA and FA caused a significant reduction by >90% in lactate efflux by N1S1 tumor cells (p < 0.01). Knockdown of Slc16a3 prevented inhibition by CA. In vivo tumors grew 30-fold in volume over 4 weeks in untreated controls. By comparison, TAE resulted in near cessation of growth (10% in 4-week time period). However, both TAE + CA and TAE + FA caused a significant reduction of tumor volumes (87 and 72%, respectively) compared to control and TAE (p < 0.05). Pathologic evaluation revealed residual tumor in the TAE group but no residual viable tumor cells in the TAE + CA and TAE + FA groups. CONCLUSION: Addition of CA or FA enhances the effectiveness of TAE therapy for HCC in part by blocking lactate efflux.
A molecular mechanism of pyruvate protection against cytotoxicity of reactive oxygen species in osteoblasts.[Pubmed:16766717]
Mol Pharmacol. 2006 Sep;70(3):925-35.
We demonstrated previously that exogenous pyruvate has a protective action against cell death by hydrogen peroxide in cultured osteoblasts through a mechanism associated with its antioxidative property. In the present study, we have evaluated possible participation of monocarboxylate transporters (MCTs) responsible for the bidirectional membrane transport of pyruvate in the cytoprotective property in osteoblasts. Expression of the MCT2 isoform was found in cultured rat calvarial osteoblasts and in osteoblasts located on mouse tibia at both mRNA and protein levels. The accumulation of [14C]pyruvate occurred in a temperature- and pH-dependent manner in osteoblasts cultured for 7 days with high sensitivity to a specific MCT inhibitor, whereas pyruvate was released into extracellular spaces from cultured osteoblasts in a fashion sensitive to the MCT inhibitor. Transient overexpression of the MCT2 isoform led to reduced vulnerability to the cytotoxicity of hydrogen peroxide with an increased activity of [14C]pyruvate accumulation in murine osteoblastic MC3T3-E1 cells. Ovariectomy significantly decreased the content of pyruvate in femoral bone marrows in mice in vivo, whereas daily i.p. administration of pyruvate at 0.25 g/kg significantly prevented alterations of several histomorphometric parameters as well as cancellous bone loss in femurs by ovariectomy on 28 days after the operation. These results suggest that MCTs may be functionally expressed by osteoblasts to play a pivotal role in mechanisms related to the cytoprotective property of pyruvate.
Identification of the mitochondrial pyruvate carrier in Saccharomyces cerevisiae.[Pubmed:12887330]
Biochem J. 2003 Sep 15;374(Pt 3):607-11.
Mitochondrial pyruvate transport is fundamental for metabolism and mediated by a specific inhibitable carrier. We have identified the yeast mitochondrial pyruvate carrier by measuring inhibitor-sensitive pyruvate uptake into mitochondria from 18 different Saccharomyces cerevisiae mutants, each lacking an unattributed member of the mitochondrial carrier family (MCF). Only mitochondria from the YIL006w deletion mutant exhibited no inhibitor-sensitive pyruvate transport, but otherwise behaved normally. YIL006w encodes a 41.9 kDa MCF member with homologous proteins present in both the human and mouse genomes.
The inhibition of pyruvate transport across the plasma membrane of the bloodstream form of Trypanosoma brucei and its metabolic implications.[Pubmed:8526859]
Biochem J. 1995 Dec 1;312 ( Pt 2):479-84.
The pyruvate produced by glycolysis in the bloodstream form of the trypanosome is excreted into the host bloodstream by a facilitated diffusion carrier. The sensitivity of pyruvate transport for alpha-cyano-4-hydroxycinnamate and the compound UK5099 [alpha-cyano-beta-(1-phenylindol-3-yl)acrylate], which are known to be selective inhibitors of pyruvate (monocarboxylate) transporters present in mitochondria and the plasma membrane of eukaryotic cells, was examined. The trypanosomal pyruvate carrier was found to be rather insensitive to inhibition by alpha-cyano-4-hydroxycinnamate (Ki = 17 mM) but could be completely blocked by UK5099 (Ki = 49 microM). Inhibition of pyruvate transport resulted in the retention, and concomitant accumulation, of pyruvate within the trypanosomes, causing acidification of the cytosol and osmotic destabilization of the cells. Our results indicate that this physiological state has serious metabolic consequences and ultimately leads to cell death; thereby identifying the pyruvate carrier as a possible target for chemotherapeutic intervention.


