Metoprolol Tartrateβ1-adrenergic blocking agent CAS# 56392-17-7 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 56392-17-7 | SDF | Download SDF |
| PubChem ID | 441308 | Appearance | Powder |
| Formula | C34H56N2O12 | M.Wt | 684.81 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
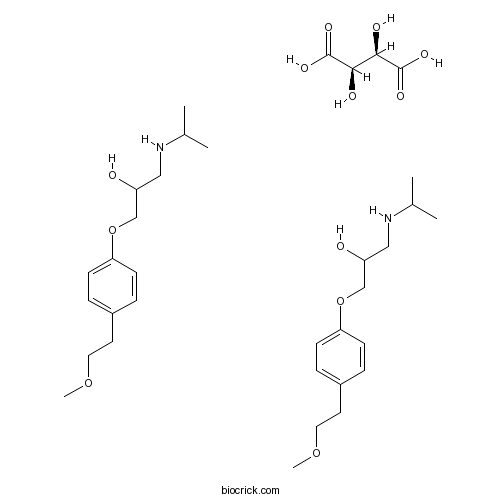
| Chemical Name | (2R,3R)-2,3-dihydroxybutanedioic acid;1-[4-(2-methoxyethyl)phenoxy]-3-(propan-2-ylamino)propan-2-ol | ||
| SMILES | CC(C)NCC(COC1=CC=C(C=C1)CCOC)O.CC(C)NCC(COC1=CC=C(C=C1)CCOC)O.C(C(C(=O)O)O)(C(=O)O)O | ||
| Standard InChIKey | YGULWPYYGQCFMP-CEAXSRTFSA-N | ||
| Standard InChI | InChI=1S/2C15H25NO3.C4H6O6/c2*1-12(2)16-10-14(17)11-19-15-6-4-13(5-7-15)8-9-18-3;5-1(3(7)8)2(6)4(9)10/h2*4-7,12,14,16-17H,8-11H2,1-3H3;1-2,5-6H,(H,7,8)(H,9,10)/t;;1-,2-/m..1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective β1-adrenoceptor antagonist (Ki values are 47, 2960 and 10100 nM for β1, β2 and β3 adrenoceptors respectively). Inhibits spontaneous endothelin-1 production in vitro and displays antihypertensive and antianginal activity in vivo. |

Metoprolol Tartrate Dilution Calculator

Metoprolol Tartrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4603 mL | 7.3013 mL | 14.6026 mL | 29.2052 mL | 36.5065 mL |
| 5 mM | 0.2921 mL | 1.4603 mL | 2.9205 mL | 5.841 mL | 7.3013 mL |
| 10 mM | 0.146 mL | 0.7301 mL | 1.4603 mL | 2.9205 mL | 3.6506 mL |
| 50 mM | 0.0292 mL | 0.146 mL | 0.2921 mL | 0.5841 mL | 0.7301 mL |
| 100 mM | 0.0146 mL | 0.073 mL | 0.146 mL | 0.2921 mL | 0.3651 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Metoprolol is a cardioselective β1-adrenergic blocking agent.Patients took 50 mg metoprolol twice daily with weekly titration to response or 200 mg twice daily. beta(1)-adrenergic receptor polymorphisms are important determinants of antihypertensive respo
- Netilmicin Sulfate
Catalog No.:BCC4683
CAS No.:56391-57-2
- Epirubicin HCl
Catalog No.:BCC1192
CAS No.:56390-09-1
- Tirotundin
Catalog No.:BCN5754
CAS No.:56377-67-4
- Capillarisin
Catalog No.:BCN2461
CAS No.:56365-38-9
- Oxybutynin
Catalog No.:BCC3833
CAS No.:5633-20-5
- Hedychenone
Catalog No.:BCN5753
CAS No.:56324-54-0
- Conocarpan acetate
Catalog No.:BCN7584
CAS No.:56319-04-1
- Moracin M
Catalog No.:BCN3292
CAS No.:56317-21-6
- 5,6,7,8-Tetramethoxycoumarin
Catalog No.:BCN5752
CAS No.:56317-15-8
- Kaempferol 3-O-(6''-galloyl)-beta-D-glucopyranoside
Catalog No.:BCN1416
CAS No.:56317-05-6
- Trimethylapigenin
Catalog No.:BCN8081
CAS No.:5631-70-9
- 3-(2,4-Dihydroxyphenyl)propionic acid
Catalog No.:BCN5751
CAS No.:5631-68-5
- UK-5099
Catalog No.:BCC2021
CAS No.:56396-35-1
- Hop-17(21)-en-3-ol
Catalog No.:BCN5755
CAS No.:564-14-7
- Sclareolide
Catalog No.:BCC6492
CAS No.:564-20-5
- Doxycycline
Catalog No.:BCN2397
CAS No.:564-25-0
- 11α-Hydroxyandrost-4-ene-3,17-dione
Catalog No.:BCC8432
CAS No.:564-33-0
- Hinokiol
Catalog No.:BCN5759
CAS No.:564-73-8
- Tetrahydrolachnophyllum lactone
Catalog No.:BCN4759
CAS No.:56407-87-5
- Methyl eichlerianate
Catalog No.:BCN5756
CAS No.:56421-12-6
- Eichlerianic acid
Catalog No.:BCN5757
CAS No.:56421-13-7
- 4,9-Dihydroxy-alpha-lapachone
Catalog No.:BCN5758
CAS No.:56473-67-7
- Steppogenin
Catalog No.:BCN5760
CAS No.:56486-94-3
- Angelic acid
Catalog No.:BCN3410
CAS No.:565-63-9
Sublingual fast dissolving niosomal films for enhanced bioavailability and prolonged effect of metoprolol tartrate.[Pubmed:27536063]
Drug Des Devel Ther. 2016 Aug 2;10:2421-33.
The aim of the present work was to prepare and evaluate sublingual fast dissolving films containing Metoprolol Tartrate-loaded niosomes. Niosomes were utilized to allow for prolonged release of the drug, whereas the films were used to increase the drug's bioavailability via the sublingual route. Niosomes were prepared using span 60 and cholesterol at different drug to surfactant ratios. The niosomes were characterized for size, zeta-potential, and entrapment efficiency. The selected niosomal formulation was incorporated into polymeric films using hydroxypropyl methyl cellulose E15 and methyl cellulose as film-forming polymers and Avicel as superdisintegrant. The physical characteristics (appearance, texture, pH, uniformity of weight and thickness, disintegration time, and palatability) of the prepared films were studied, in addition to evaluating the in vitro drug release, stability, and in vivo pharmacokinetics in rabbits. The release of the drug from the medicated film was fast (99.9% of the drug was released within 30 minutes), while the drug loaded into the niosomes, either incorporated into the film or not, showed only 22.85% drug release within the same time. The selected sublingual film showed significantly higher rate of drug absorption and higher drug plasma levels compared with that of commercial oral tablet. The plasma levels remained detectable for 24 hours following sublingual administration, compared with only 12 hours after administration of the oral tablet. In addition, the absolute bioavailability of the drug (ie, relative to intravenous administration) following sublingual administration was found to be significantly higher (91.06%+/-13.28%), as compared with that after oral tablet administration (39.37%+/-11.4%). These results indicate that the fast dissolving niosomal film could be a promising delivery system to enhance the bioavailability and prolong the therapeutic effect of Metoprolol Tartrate.
Older adults with heart failure treated with carvedilol, bisoprolol, or metoprolol tartrate: risk of mortality.[Pubmed:27859924]
Pharmacoepidemiol Drug Saf. 2017 Jan;26(1):81-90.
PURPOSE: The long-term use of beta-blockers has been shown to improve clinical outcomes among patients with heart failure (HF). However, a lack of data persists in assessing whether carvedilol or bisoprolol are superior to Metoprolol Tartrate in clinical practice. We endeavored to compare the effectiveness of beta-blockers among older adults following a primary hospital admission for HF. METHODS: We conducted a cohort study using Quebec administrative databases to identify patients who were using beta-blockers, carvedilol, bisoprolol, or Metoprolol Tartrate after the diagnosis of HF. We characterized the patients by the type of beta-blocker prescribed at discharge of their first HF hospitalization. An adjusted multivariate Cox proportional hazards model was used to compare the primary outcome of all-cause mortality. We also conducted analyses by matching for a propensity score for initiation of beta-blocker therapy and assessed the effect on primary outcome. RESULTS: Among 3197 patients with HF with a median follow-up of 2.8 years, the crude annual mortality rates (per 100 person-years) were at 16, 14.9, and 17.7 for Metoprolol Tartrate, carvedilol, and bisoprolol, respectively. Adjusted hazard ratios of carvedilol (hazard ratio 0.92; 0.78-1.09) and bisoprolol (hazard ratio 1.04; 0.93-1.16) were not significantly different from that of Metoprolol Tartrate in improving survival. After matching for propensity score, carvedilol and bisoprolol showed no additional benefit with respect to all-cause mortality compared with Metoprolol Tartrate. CONCLUSIONS: Our evidence suggests no differential effect of beta-blockers on all-cause mortality among older adults with HF. Copyright (c) 2016 John Wiley & Sons, Ltd.
Preparation and evaluation of metoprolol tartrate sustained-release pellets using hot melt extrusion combined with hot melt coating.[Pubmed:28128647]
Drug Dev Ind Pharm. 2017 Jun;43(6):939-946.
The objective of this study was to prepare and evaluate Metoprolol Tartrate sustained-release pellets. Cores were prepared by hot melt extrusion and coated pellets were prepared by hot melt coating. Cores were found to exist in a single-phase state and drug in amorphous form. Plasticizers had a significant effect on torque and drug content, while release modifiers and coating level significantly affected the drug-release behavior. The mechanisms of drug release from cores and coated pellets were Fickian diffusion and diffusion-erosion. The coated pellets exhibited sustained-release properties in vitro and in vivo.
S-metoprolol: the 2008 clinical review.[Pubmed:18828349]
J Indian Med Assoc. 2008 Apr;106(4):259-62.
Metoprolol is a widely used cardioselective beta-blocker. However, like all other beta-blockers it is also a racemic mixture of R- and S- isomers. The beta 1 blocking activity (cardioselectivity) of metoprolol resides in S-isomer while R-isomer exhibits beta 2 blocking activity. As both these isomers have different pharmacological properties, racemic metoprolol can be considered a combination of two different drugs in a fixed 1:1 ratio. The needless administration of the non beta-blocking R-enantiomer that makes up 50% of racemate actually puts the patient at an increased risk of side-effects, drug interactions and loss of cardioselectivity with up-titration of dosing. Clinical experience with chirally pure S-metoprolol at half the dose of racemate has shown it to be as effective as racemate in the treatment of patients with hypertension and angina. S-metoprolol has been shown to be effective and well-tolerated in patients with coexisting diabetes, COPD, and hyperlipidaemia. This confirms higher cardioselectivity of S-metoprolol in clinical settings. Less interaction potential of S-metoprolol compared to R-isomer further makes it a sensible choice in patients taking CYP2D6 inhibitors or in patients with heart failure or hepatic insufficiency. This article reviews differing properties of two isomers of metoprolol with focus on clinical experience with S-metoprolol.
Beta-blockers reduce the release and synthesis of endothelin-1 in human endothelial cells.[Pubmed:10092983]
Eur J Clin Invest. 1999 Jan;29(1):12-6.
BACKGROUND: Endothelins play an important role in cardiovascular diseases, and clinical trials have shown a reduction in endothelin levels after long-term treatment of chronic heart failure with beta-adrenergic antagonists. It is not known, however, whether this effect is caused by haemodynamic changes associated with the use of beta-adrenergic antagonists or by direct interaction of beta-blockers with human endothelial cells. The aim of this study was to determine whether beta-adrenergic antagonists have an influence on endothelin-1 (ET-1) synthesis and release in human endothelial cells. METHODS: Pretreatment of cultured endothelial cells from human umbilical veins (HUVECs) with different concentrations of the non-selective beta-blocker propranolol, the beta 1-blocker metoprolol and the beta 1-blocker and beta 2-agonist celiprolol (all 10(-7)-10(-4) mol L-1) was found to reduce ET-1 production. This ET-1-reducing effect was even more pronounced in thrombin-stimulated cells (10(-5) mol L-1 of propranolol, metoprolol and celiprolol: 19% +/- 5.8%, 25% +/- 4% and 37% +/- 5.2% respectively). RESULTS: Quantitative reverse transcriptase polymerase chain reaction and Northern blotting confirmed an inhibitory effect of the beta-blocker on biosynthesis. Furthermore, the ET-1-reducing effect of propranolol, metoprolol and celiprolol was not due to a compensatory increase in prostacyclin and was not reversible by N-nitro-L-arginine. CONCLUSION: The effect of beta-adrenergic antagonists on ET-1 production of the endothelium may at least partially explain the efficacy of beta-blockers in the treatment of diseases such as advanced heart failure, essential hypertension as well as acute coronary syndromes.


