3-(2,4-Dihydroxyphenyl)propionic acidCAS# 5631-68-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5631-68-5 | SDF | Download SDF |
| PubChem ID | 96384 | Appearance | Powder |
| Formula | C9H10O4 | M.Wt | 182.2 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-(2,4-dihydroxyphenyl)propanoic acid | ||
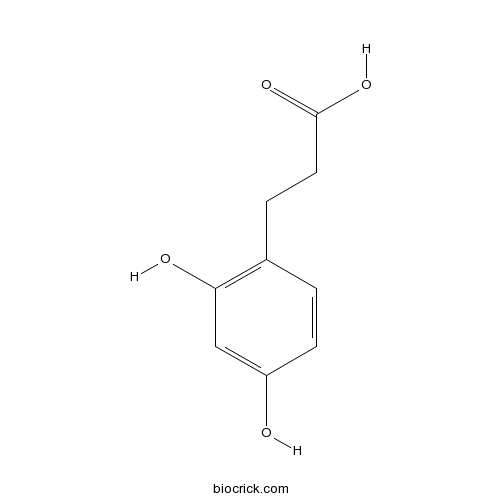
| SMILES | C1=CC(=C(C=C1O)O)CCC(=O)O | ||
| Standard InChIKey | HMCMTJPPXSGYJY-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 3-(2,4-Dihydroxyphenyl)propionic acid and l-ascorbic acid show tyrosinase inhibition activity, a synergistic effect on tyrosinase inhibition was observed when the two compounds were mixed. |
| Targets | Tyrosinase |
| In vitro | Prevention of Agaricus bisporus postharvest browning with tyrosinase inhibitors[Reference: WebLink]Postharvest Biology and Technology, 2006,39(3):272-7.Postharvest browning of Agaricus bisporus mushrooms is a severe problem that reduces the shelf life of harvested mushrooms. Mushroom browning occurs mainly as a result of tyrosinase activity, an enzyme belonging to the polyphenol oxidase (PPO) family and known to be a key enzyme in melanin biosynthesis.
|
| Kinase Assay | The evaluation of the synergistic effect of 3-(2,4-dihydroxyphenyl)propionic acid and l-ascorbic acid on tyrosinase inhibition.[Pubmed: 27442367 ]Z Naturforsch C. 2017 Mar 1;72(3-4):119-121.3-(2,4-Dihydroxyphenyl)propionic acid (DDPA) and l-ascorbic acid (vitamin C) show tyrosinase inhibition activity. A synergistic effect on tyrosinase inhibition was observed when the two compounds were mixed. The effect significantly decreased the IC50 value of both compounds. |

3-(2,4-Dihydroxyphenyl)propionic acid Dilution Calculator

3-(2,4-Dihydroxyphenyl)propionic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4885 mL | 27.4424 mL | 54.8847 mL | 109.7695 mL | 137.2119 mL |
| 5 mM | 1.0977 mL | 5.4885 mL | 10.9769 mL | 21.9539 mL | 27.4424 mL |
| 10 mM | 0.5488 mL | 2.7442 mL | 5.4885 mL | 10.9769 mL | 13.7212 mL |
| 50 mM | 0.1098 mL | 0.5488 mL | 1.0977 mL | 2.1954 mL | 2.7442 mL |
| 100 mM | 0.0549 mL | 0.2744 mL | 0.5488 mL | 1.0977 mL | 1.3721 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fluoxetine HCl
Catalog No.:BCC1191
CAS No.:56296-78-7
- Aloperine
Catalog No.:BCN8466
CAS No.:56293-29-9
- Uncarine C
Catalog No.:BCC8261
CAS No.:5629-60-7
- Anigorufone
Catalog No.:BCN7171
CAS No.:56252-32-5
- 2-O-Methylanigorufone
Catalog No.:BCN7180
CAS No.:56252-05-2
- Hydroxyanigorufone
Catalog No.:BCN7184
CAS No.:56252-02-9
- Cyclo(Leu-Val)
Catalog No.:BCN2436
CAS No.:5625-50-3
- Cyclo(Tyr-Gly)
Catalog No.:BCN2414
CAS No.:5625-49-0
- Porson
Catalog No.:BCN5750
CAS No.:56222-03-8
- Methyl lycernuate A
Catalog No.:BCN5749
CAS No.:56218-46-3
- Torsemide
Catalog No.:BCC4871
CAS No.:56211-40-6
- Terpinine-4-ol
Catalog No.:BCN8250
CAS No.:562-74-3
- Trimethylapigenin
Catalog No.:BCN8081
CAS No.:5631-70-9
- Kaempferol 3-O-(6''-galloyl)-beta-D-glucopyranoside
Catalog No.:BCN1416
CAS No.:56317-05-6
- 5,6,7,8-Tetramethoxycoumarin
Catalog No.:BCN5752
CAS No.:56317-15-8
- Moracin M
Catalog No.:BCN3292
CAS No.:56317-21-6
- Conocarpan acetate
Catalog No.:BCN7584
CAS No.:56319-04-1
- Hedychenone
Catalog No.:BCN5753
CAS No.:56324-54-0
- Oxybutynin
Catalog No.:BCC3833
CAS No.:5633-20-5
- Capillarisin
Catalog No.:BCN2461
CAS No.:56365-38-9
- Tirotundin
Catalog No.:BCN5754
CAS No.:56377-67-4
- Epirubicin HCl
Catalog No.:BCC1192
CAS No.:56390-09-1
- Netilmicin Sulfate
Catalog No.:BCC4683
CAS No.:56391-57-2
- Metoprolol Tartrate
Catalog No.:BCC4330
CAS No.:56392-17-7
The evaluation of the synergistic effect of 3-(2,4-dihydroxyphenyl)propionic acid and l-ascorbic acid on tyrosinase inhibition.[Pubmed:27442367]
Z Naturforsch C. 2017 Mar 1;72(3-4):119-121.
3-(2,4-Dihydroxyphenyl)propionic acid (DDPA) and l-ascorbic acid (vitamin C) show tyrosinase inhibition activity. A synergistic effect on tyrosinase inhibition was observed when the two compounds were mixed. The effect significantly decreased the IC50 value of both compounds.


