TorsemideCAS# 56211-40-6 |
- AZD8055
Catalog No.:BCC3629
CAS No.:1009298-09-2
- BEZ235 Tosylate
Catalog No.:BCC1416
CAS No.:1028385-32-1
- WYE-354
Catalog No.:BCC1059
CAS No.:1062169-56-5
- VS-5584 (SB2343)
Catalog No.:BCC2047
CAS No.:1246560-33-7
- XL388
Catalog No.:BCC2059
CAS No.:1251156-08-7
- Rapamycin (Sirolimus)
Catalog No.:BCC3592
CAS No.:53123-88-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 56211-40-6 | SDF | Download SDF |
| PubChem ID | 41781 | Appearance | Powder |
| Formula | C16H20N4O3S | M.Wt | 348.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Torasemide | ||
| Solubility | DMSO : ≥ 3.5 mg/mL (10.05 mM) *"≥" means soluble, but saturation unknown. | ||
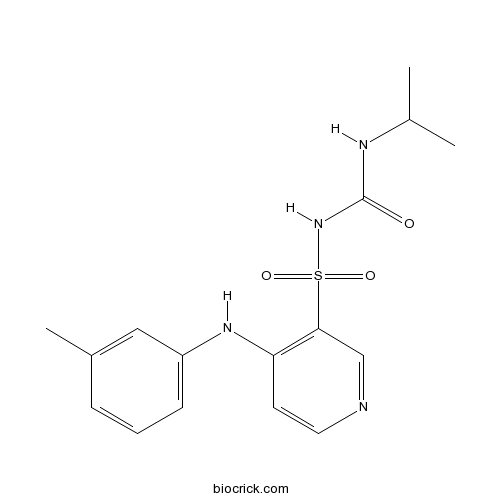
| Chemical Name | 1-[4-(3-methylanilino)pyridin-3-yl]sulfonyl-3-propan-2-ylurea | ||
| SMILES | CC(C)NC(=O)N[S](=O)(=O)c1cnccc1Nc2cccc(C)c2 | ||
| Standard InChIKey | NGBFQHCMQULJNZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H20N4O3S/c1-11(2)18-16(21)20-24(22,23)15-10-17-8-7-14(15)19-13-6-4-5-12(3)9-13/h4-11H,1-3H3,(H,17,19)(H2,18,20,21) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Torsemide is a pyridine-sulfonyl urea type loop diuretic.
Target: Others
Torasemide is a pyridine-sulfonylurea type loop diuretic mainly used in the management of edema associated with congestive heart failure. It is also used at low doses for the management of hypertension. Torsemide significantly reduced total HF readmissions (relative risk [RR]: 0.41, 95% CI: 0.28-0.61, p < 0.0001) and HF readmissions (RR: 0.53, 95% CI: 0.33-0.84, p = 0.008) as well as CV readmissions (RR: 0.77, 95% CI: 0.60-0.98, p = 0.03) in patients with at least 1 readmission. Torsemide caused a 14% reduction in all-cause mortality (RR: 0.86 [0.53-1.39], p = 0.54). Torsemide significantly reduces HF and CV-related hospital readmissions in systolic HF. Furthermore, torsemide is associated with a trend in reducing all-cause mortality [1]. Torsemide has several characteristics that make it suitable for treatment of advanced heart failure including longer half-life, increased potency of diuretic action, and anti-aldosterone effects. This case report details the administration of torsemide in 3 dogs with advanced heart failure and apparent furosemide resistance [2]. References: | |||||

Torsemide Dilution Calculator

Torsemide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8701 mL | 14.3505 mL | 28.701 mL | 57.402 mL | 71.7525 mL |
| 5 mM | 0.574 mL | 2.8701 mL | 5.7402 mL | 11.4804 mL | 14.3505 mL |
| 10 mM | 0.287 mL | 1.435 mL | 2.8701 mL | 5.7402 mL | 7.1752 mL |
| 50 mM | 0.0574 mL | 0.287 mL | 0.574 mL | 1.148 mL | 1.435 mL |
| 100 mM | 0.0287 mL | 0.1435 mL | 0.287 mL | 0.574 mL | 0.7175 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Torsemide is a pyridine-sulfonyl urea type loop diuretic.
- Terpinine-4-ol
Catalog No.:BCN8250
CAS No.:562-74-3
- CGP 13501
Catalog No.:BCC7097
CAS No.:56189-68-5
- Acarbose
Catalog No.:BCC1190
CAS No.:56180-94-0
- H-Sar-OtBu.HCl
Catalog No.:BCC3336
CAS No.:5616-81-9
- Valrubicin
Catalog No.:BCC5219
CAS No.:56124-62-0
- 4-O-Methylhelichrysetin
Catalog No.:BCN3986
CAS No.:56121-44-9
- Asperglaucide
Catalog No.:BCN5748
CAS No.:56121-42-7
- Parisaponin I
Catalog No.:BCN2835
CAS No.:561007-63-4
- Securinine
Catalog No.:BCN6988
CAS No.:5610-40-2
- Ionomycin calcium salt
Catalog No.:BCC5805
CAS No.:56092-82-1
- Ionomycin free acid
Catalog No.:BCC7261
CAS No.:56092-81-0
- 3-Methoxyshancigusin I
Catalog No.:BCC9002
CAS No.:
- Methyl lycernuate A
Catalog No.:BCN5749
CAS No.:56218-46-3
- Porson
Catalog No.:BCN5750
CAS No.:56222-03-8
- Cyclo(Tyr-Gly)
Catalog No.:BCN2414
CAS No.:5625-49-0
- Cyclo(Leu-Val)
Catalog No.:BCN2436
CAS No.:5625-50-3
- Hydroxyanigorufone
Catalog No.:BCN7184
CAS No.:56252-02-9
- 2-O-Methylanigorufone
Catalog No.:BCN7180
CAS No.:56252-05-2
- Anigorufone
Catalog No.:BCN7171
CAS No.:56252-32-5
- Uncarine C
Catalog No.:BCC8261
CAS No.:5629-60-7
- Aloperine
Catalog No.:BCN8466
CAS No.:56293-29-9
- Fluoxetine HCl
Catalog No.:BCC1191
CAS No.:56296-78-7
- 3-(2,4-Dihydroxyphenyl)propionic acid
Catalog No.:BCN5751
CAS No.:5631-68-5
- Trimethylapigenin
Catalog No.:BCN8081
CAS No.:5631-70-9
Torsemide Versus Furosemide in Patients With Acute Heart Failure (from the ASCEND-HF Trial).[Pubmed:26704029]
Am J Cardiol. 2016 Feb 1;117(3):404-11.
Furosemide is the most commonly used loop diuretic in patients with heart failure (HF) despite data suggesting potential pharmacologic and antifibrotic benefits with Torsemide. We investigated patients with HF in Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure who were discharged on either Torsemide or furosemide. Using inverse probability weighting to account for the nonrandom selection of diuretic, we assessed the relation between choice of diuretic at discharge with 30-day mortality or HF hospitalization and 180-day mortality. Of 7,141 patients in the trial, 4,177 patients were included in this analysis, of which 87% (n = 3,620) received furosemide and 13% (n = 557) received Torsemide. Torsemide-treated patients had lower ejection fraction and blood pressure and higher creatinine and natriuretic peptide level compared with furosemide. Torsemide was associated with similar outcomes on unadjusted analysis and nominally lower events on adjusted analysis (30-day mortality/HF hospitalization odds ratio 0.89, 95% CI 0.62 to 1.29, p = 0.55 and 180-day mortality hazard ratio 0.86, 95% CI 0.63 to 1.19, p = 0.37). In conclusion, these data are hypothesis-generating and randomized comparative effectiveness trials are needed to investigate the optimal diuretic choice.
Comparative effectiveness of torsemide versus furosemide in heart failure patients: insights from the PROTECT trial.[Pubmed:26403536]
Future Cardiol. 2015 Sep;11(5):585-95.
AIM: The authors assessed the comparative effectiveness of Torsemide versus furosemide in the PROTECT trial. METHODS: The authors assessed the relationship between loop diuretic at discharge and death or cardiovascular/renal hospitalization within 30 days, and death through 150 days postdischarge using inverse probability weighting. RESULTS: Out of 1004 patients, 83.5% received furosemide and 16.5% Torsemide. Torsemide patients had higher blood urea nitrogen, and more in-hospital worsening heart failure. Following adjustment, Torsemide was associated with similar 30-day outcomes compared with furosemide (p = 0.93), but remained associated with increased 150-day death (hazard ratio: 2.26; 95% CI: 1.40-3.66; p < 0.001). CONCLUSION: Patients treated with Torsemide had features of greater disease severity, similar 30-day outcomes but increased 150-day mortality. Prospective randomized trials are needed to investigate the effect of Torsemide versus furosemide.
Torsemide Fast Dissolving Tablets: Development, Optimization Using Box-Bhenken Design and Response Surface Methodology, In Vitro Characterization, and Pharmacokinetic Assessment.[Pubmed:28050711]
AAPS PharmSciTech. 2017 Aug;18(6):2168-2179.
The present study planed to develop new fast dissolving tablets (FDTs) of Torsemide. Solid dispersions (SDs) of Torsemide and sorbitol (3:1) or polyvinylpyrrolidone (PVP) k25 were prepared. The prepared SDs were evaluated for in-vitro dissolution. Fourier transform infrared spectroscopy and differential scanning calorimetry for SDs revealed no drug/excipient interactions and transformation of Torsemide to the amorphous form. Torsemide/sorbitol SD was selected for formulation of Torsemide FDTs by direct compression method. Box-Bhenken factorial design was employed to design 15 formulations using croscarmellose sodium and crospovidone at different concentrations. The response surface methodology was used to analyze the effect of changing these concentrations (independent variables) on disintegration time (Y1), percentage friability (Y2), and amount Torsemide released at 10 min. The physical mixtures of Torsemide and the used excipients were evaluated for angle of repose, Hausner's ratio, and Carr's index. The prepared FDTs tablets were evaluated for wetting and disintegration time, weight variation, drug content, percentage friability, thickness, hardness, and in vitro release. Based on the in-vitro results and factorial design characterization, F10 and F7 were selected for bioavailability studies following administration to Albino New Zealand rabbits. They showed significantly higher C max and (AUC0-12) and shorter T max than those obtained after administration of the corresponding ordinary commercial Torseretic (R) tablets. Stability study was conducted for F10 that showed good stability upon storage at 30 degrees C/75% RH and 40 degrees C/75% RH for 3 months.
Effect of aliskiren, telmisartan and torsemide on cardiac dysfunction in l-nitro arginine methyl ester (l-NAME) induced hypertension in rats.[Pubmed:26644935]
J Adv Res. 2015 Nov;6(6):967-74.
Comparative study of cardio protective effect of aliskiren, telmisartan, and Torsemide was carried out on l-nitro arginine methyl ester (l-NAME) induced hypertension in rats. The three drugs were given daily for 8 weeks simultaneously with l-NAME, with a control group for each drug and l-NAME. The degree of protection was assessed by measurement of systolic blood pressure and heart rate of animals every two weeks. At the end of the experimental period blood sampling was carried out for estimation of the level of NO2 (-)/NO3 (-). After which animals were sacrificed for heart dissection to detect collagen types I and III gene expression. Histopathological study was done to evaluate the extension of collagen deposits. The study revealed that the three drugs decreased blood pressure significantly compared to l-NAME. There was no significant difference between aliskiren and telmisartan in all measurements, but there was significant decrease in measurements of both aliskiren and telmisartan treated groups compared to Torsemide starting from 4th week. There were insignificant changes in pulse rate values between the three l-NAME treated groups through the experiment. The three drugs significantly increased NO compared to l-NAME. Collagen I and III gene expression was significantly decreased by the three drugs but the highest percentage of inhibition was with telmisartan compared to l-NAME. Comparing the percentage inhibition of cardiac fibrosis, there was insignificant difference between telmisartan and Torsemide treated groups while both were superior to aliskiren. In conclusion, further experimental studies are required to elucidate the potential cardioprotective mechanisms of aliskiren, telmisartan and Torsemide, and assess their efficacy in treatment of heart failure.


