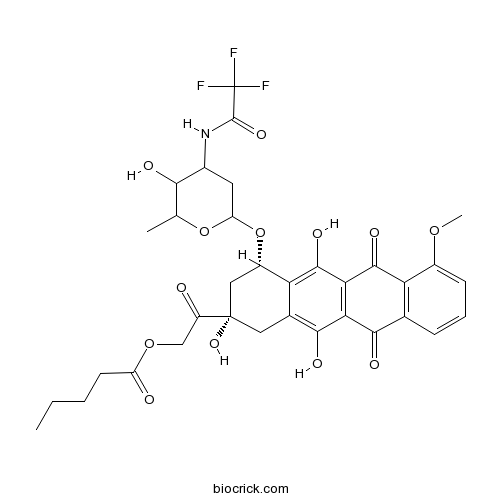
ValrubicinTopoisomerase inhibitor CAS# 56124-62-0 |
- Resminostat hydrochloride
Catalog No.:BCC1888
CAS No.:1187075-34-8
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- Pyroxamide
Catalog No.:BCC2424
CAS No.:382180-17-8
- LAQ824 (NVP-LAQ824,Dacinostat)
Catalog No.:BCC2160
CAS No.:404951-53-7
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
- KD 5170
Catalog No.:BCC2420
CAS No.:940943-37-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 56124-62-0 | SDF | Download SDF |
| PubChem ID | 41744 | Appearance | Powder |
| Formula | C34H36F3NO13 | M.Wt | 723.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AD-32 | ||
| Solubility | DMSO : ≥ 130 mg/mL (179.65 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [2-oxo-2-[(2S,4S)-2,5,12-trihydroxy-4-[5-hydroxy-6-methyl-4-[(2,2,2-trifluoroacetyl)amino]oxan-2-yl]oxy-7-methoxy-6,11-dioxo-3,4-dihydro-1H-tetracen-2-yl]ethyl] pentanoate | ||
| SMILES | CCCCC(=O)OCC(=O)C1(CC(C2=C(C3=C(C(=C2C1)O)C(=O)C4=C(C3=O)C(=CC=C4)OC)O)OC5CC(C(C(O5)C)O)NC(=O)C(F)(F)F)O | ||
| Standard InChIKey | ZOCKGBMQLCSHFP-ZQUOIQDWSA-N | ||
| Standard InChI | InChI=1S/C34H36F3NO13/c1-4-5-9-21(40)49-13-20(39)33(47)11-16-24(19(12-33)51-22-10-17(27(41)14(2)50-22)38-32(46)34(35,36)37)31(45)26-25(29(16)43)28(42)15-7-6-8-18(48-3)23(15)30(26)44/h6-8,14,17,19,22,27,41,43,45,47H,4-5,9-13H2,1-3H3,(H,38,46)/t14?,17?,19-,22?,27?,33-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Valrubicin Dilution Calculator

Valrubicin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3819 mL | 6.9095 mL | 13.819 mL | 27.6381 mL | 34.5476 mL |
| 5 mM | 0.2764 mL | 1.3819 mL | 2.7638 mL | 5.5276 mL | 6.9095 mL |
| 10 mM | 0.1382 mL | 0.691 mL | 1.3819 mL | 2.7638 mL | 3.4548 mL |
| 50 mM | 0.0276 mL | 0.1382 mL | 0.2764 mL | 0.5528 mL | 0.691 mL |
| 100 mM | 0.0138 mL | 0.0691 mL | 0.1382 mL | 0.2764 mL | 0.3455 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Valrubicin is a chemotherapy agent, inhibits TPA- and PDBu-induced PKC activation with IC50s of 0.85 and 1.25 μM, respectively, and has antitumor and antiinflammatory activity.
In Vitro:Valrubicin (AD 32) is a chemotherapy agent, inhibits TPA- and PDBu-induced PKC activation with IC50s of 0.85 and 1.25 μM, respectively. Valrubicin inhibits the binding of [3H]PDBu to PKC. Therefore, Valrubicin competes with the tumor promoter for the PKC binding site and prevents the latter from both interacting with the phospholipid and binding to PKC[1]. Valrubicin shows cytotoxic activity against squamous cell carcinoma (SCC) cell line colony formation, with IC50s and IC90s of 8.24 ± 1.60 μM and 14.81 ± 2.82 μM for UMSCC5 cells, 15.90 ± 0.90 μM, 29.84 ± 0.84 μM for UMSCC5/CDDP‡ cells, and 10.50 ± 2.39 μM, 19.00 ± 3.91 μM for UMSCC10b cells, respectively. Moreover, Valrubicin in combination with radiation enhances the cytotoxicity[2].
In Vivo:Valrubicin (3, 6, or 9 mg) reduces tumor growth at week 3 by intratumoral jection in hamster. Valrubicin (6 mg) combined with minimally cytotoxic irradiation (150, 250, or 350 cGy) causes significant tumor shrinkage in hamster[2]. Valrubicin (0.1 μg/μL) significantly reduces the number of infiltrating neutrophils in biopsies challenged with TPA at 24 h and attenuates chronic inflammation in mice. Valrubicin also decreases the expression levels of inflammatory cytokines in the acute model[3].
References:
[1]. Chuang LF, et al. Activation of human leukemia protein kinase C by tumor promoters and its inhibition by N-trifluoroacetyladriamycin-14-valerate (AD 32). Biochem Pharmacol. 1992 Feb 18;43(4):865-72.
[2]. Wani MK, et al. Rationale for intralesional valrubicin in chemoradiation of squamous cell carcinoma of the head and neck. Laryngoscope. 2000 Dec;110(12):2026-32.
[3]. Hauge E, et al. Topical valrubicin application reduces skin inflammation in murine models. Br J Dermatol. 2012 Aug;167(2):288-95.
- 4-O-Methylhelichrysetin
Catalog No.:BCN3986
CAS No.:56121-44-9
- Asperglaucide
Catalog No.:BCN5748
CAS No.:56121-42-7
- Parisaponin I
Catalog No.:BCN2835
CAS No.:561007-63-4
- Securinine
Catalog No.:BCN6988
CAS No.:5610-40-2
- Ionomycin calcium salt
Catalog No.:BCC5805
CAS No.:56092-82-1
- Ionomycin free acid
Catalog No.:BCC7261
CAS No.:56092-81-0
- 3-Methoxyshancigusin I
Catalog No.:BCC9002
CAS No.:
- PRIMA-1
Catalog No.:BCC2413
CAS No.:5608-24-2
- Hispidin
Catalog No.:BCN3567
CAS No.:56070-89-4
- Sucralose
Catalog No.:BCC4725
CAS No.:56038-13-2
- Eburicoic acid
Catalog No.:BCN2556
CAS No.:560-66-7
- 9-Hydroxy-4-androstene-3,17-dione
Catalog No.:BCC8802
CAS No.:560-62-3
- H-Sar-OtBu.HCl
Catalog No.:BCC3336
CAS No.:5616-81-9
- Acarbose
Catalog No.:BCC1190
CAS No.:56180-94-0
- CGP 13501
Catalog No.:BCC7097
CAS No.:56189-68-5
- Terpinine-4-ol
Catalog No.:BCN8250
CAS No.:562-74-3
- Torsemide
Catalog No.:BCC4871
CAS No.:56211-40-6
- Methyl lycernuate A
Catalog No.:BCN5749
CAS No.:56218-46-3
- Porson
Catalog No.:BCN5750
CAS No.:56222-03-8
- Cyclo(Tyr-Gly)
Catalog No.:BCN2414
CAS No.:5625-49-0
- Cyclo(Leu-Val)
Catalog No.:BCN2436
CAS No.:5625-50-3
- Hydroxyanigorufone
Catalog No.:BCN7184
CAS No.:56252-02-9
- 2-O-Methylanigorufone
Catalog No.:BCN7180
CAS No.:56252-05-2
- Anigorufone
Catalog No.:BCN7171
CAS No.:56252-32-5
Photophysical characterization of anticancer drug valrubicin in rHDL nanoparticles and its use as an imaging agent.[Pubmed:26735001]
J Photochem Photobiol B. 2016 Feb;155:60-5.
Nanoparticles are target-specific drug delivery agents that are increasingly used in cancer therapy to enhance bioavailability and to reduce off target toxicity of anti-cancer agents. Valrubicin is an anti-cancer drug, currently approved only for vesicular bladder cancer treatment because of its poor water solubility. On the other hand, Valrubicin carrying reconstituted high density lipoprotein (rHDL) nanoparticles appear ideally suited for extended applications, including systemic cancer chemotherapy. We determined selected fluorescence properties of the free (unencapsulated) drug vs. Valrubicin incorporated into rHDL nanoparticles. We have found that upon encapsulation into rHDL nanoparticles the quantum yield of Valrubicin fluorescence increased six fold while its fluorescence lifetime increased about 2 fold. Accordingly, these and potassium iodide (KI) quenching data suggest that upon incorporation, Valrubicin is localized deep in the interior of the nanoparticle, inside the lipid matrix. Fluorescence anisotropy of the rHDL Valrubicin nanoparticles was also found to be high along with extended rotational correlation time. The fluorescence of Valrubicin could also be utilized to assess its distribution upon delivery to prostate cancer (PC3) cells. Overall the fluorescence properties of the rHDL: Valrubicin complex reveal valuable novel characteristics of this drug delivery vehicle that may be particularly applicable when used in systemic (intravenous) therapy.
Fabrication of an electrochemical sensor based on carbon nanotubes modified with gold nanoparticles for determination of valrubicin as a chemotherapy drug: valrubicin-DNA interaction.[Pubmed:25687007]
Mater Sci Eng C Mater Biol Appl. 2015 Apr;49:769-775.
In this study, an electrochemical sensor was fabricated based on gold nanoparticles/ ethylenediamine/ multi-wall carbon-nanotubes modified gold electrode (AuNPs/en/MWCNTs/AuE) for determination of Valrubicin in biological samples. Valrubicin was effectively accumulated on the surface of AuNPs/en/MWCNTs/AuE and produced a pair of redox peaks at around 0.662 and 0.578V (vs. Ag/AgCl) in citrate buffer (pH4.0). The electrochemical parameters including pH, buffer, ionic strength, scan rate and size of AuNPs have been optimized. There was a good linear correlation between cathodic peak current and concentration of Valrubicin in the range of 0.5 to 80.0mumolL(-1) with the detection limit of 0.018mumolL(-1) in citrate buffer (pH4.0) and 0.1molL(-1) KCl. Finally, the constructed sensor was successfully applied for determination of Valrubicin in human urine and blood serum. In further studies, the different sequences of single stranded DNA probes have been immobilized on the surface of AuNPs decorated on MWCNTs to study the interaction of oligonucleotides with Valrubicin.
Use of intravesical valrubicin in clinical practice for treatment of nonmuscle-invasive bladder cancer, including carcinoma in situ of the bladder.[Pubmed:25276228]
Ther Adv Urol. 2014 Oct;6(5):181-91.
OBJECTIVES: The objective was to conduct a US multicenter, retrospective medical record study examining the effectiveness, safety, and patterns of use of Valrubicin for treatment of nonmuscle-invasive bladder cancer (NMIBC) by clinicians since the 2009 reintroduction of Valrubicin. METHODS: Patients >/= 18 years with NMIBC who received had one or more instillations of Valrubicin (October 2009- September 2011) were eligible. The primary endpoint was event-free survival (EFS). Safety and tolerability were also assessed. RESULTS: The medical records of 113 patients met the inclusion criteria; 100 patients (88.5%) completed Valrubicin treatment. The median age was 75 years (range 42-95 years). The median NMIBC duration was 31 months since diagnosis: 51.3% (58/113) had carcinoma in situ (CIS) alone, and 31.9% (36/113) had unspecified NMIBC. Most patients, 94.7% (107/113), had more than three Valrubicin instillations and 70.8% (80/113) completed a full course. The EFS rate (95% confidence interval) was 51.6% (40.9-61.3%), 30.4% (20.4-41.1%), and 16.4% (7.9-27.5%) at 3, 6, and 12 months, respectively. Median time to an event was 3.5 (2.5-4.0) months after the first Valrubicin instillation. Local adverse reactions (LARs) were experienced by 49.6% (56/113) of patients; most LARs were mild (93.6%). The most frequent LARs were hematuria, pollakiuria, micturition urgency, bladder spasm, and dysuria. In total, 4.4% (5/113) of patients discontinued Valrubicin because of adverse events or LARs. CONCLUSIONS: Data from the present retrospective study are consistent with previous prospective clinical trials that demonstrated Valrubicin effectiveness and tolerability for select patients with CIS, before considering cystectomy. Additional prospective studies are warranted to evaluate Valrubicin safety and efficacy in the broader patient population with NMIBC.
Valrubicin in refractory non-muscle invasive bladder cancer.[Pubmed:26569509]
Expert Rev Anticancer Ther. 2015;15(12):1379-87.
The most effective intravesical regimen for the treatment of non-muscle invasive bladder cancer (NMIBC) refractory to Bacillus Calmette-Guerin (BCG) has still not been identified or optimized to minimize recurrence-free survival and prevent progression reliably and consistently. Valrubicin, however, is a cytotoxic chemotherapeutic agent that is used as an intravesical agent for BCG-refractory carcinoma in situ (CIS) of the urinary bladder in patients for whom immediate cystectomy would be associated with unacceptable morbidity or mortality. Here we analyze the literature regarding the treatment of non-muscle invasive urothelial malignancies with intravesical Valrubicin for refractory bladder cancer, present our opinion on its current clinical impact and speculate on how its utilization will evolve in the near future.


