CGP 13501Positive modulator of GABAB receptors,allosteric CAS# 56189-68-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 56189-68-5 | SDF | Download SDF |
| PubChem ID | 3519541 | Appearance | Powder |
| Formula | C19H30O2 | M.Wt | 290.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in ethanol and to 100 mM in DMSO | ||
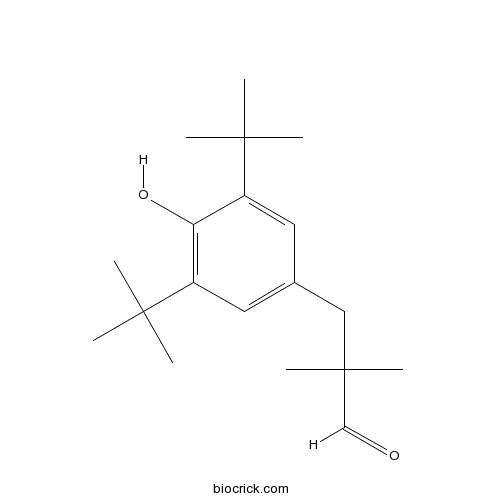
| Chemical Name | 3-(3,5-ditert-butyl-4-hydroxyphenyl)-2,2-dimethylpropanal | ||
| SMILES | CC(C)(C)C1=CC(=CC(=C1O)C(C)(C)C)CC(C)(C)C=O | ||
| Standard InChIKey | XGWATTXMMMANFJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H30O2/c1-17(2,3)14-9-13(11-19(7,8)12-20)10-15(16(14)21)18(4,5)6/h9-10,12,21H,11H2,1-8H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Positive allosteric modulator of GABAB receptors, potentiating the effects of GABA at this receptor type. |

CGP 13501 Dilution Calculator

CGP 13501 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4431 mL | 17.2153 mL | 34.4305 mL | 68.861 mL | 86.0763 mL |
| 5 mM | 0.6886 mL | 3.4431 mL | 6.8861 mL | 13.7722 mL | 17.2153 mL |
| 10 mM | 0.3443 mL | 1.7215 mL | 3.4431 mL | 6.8861 mL | 8.6076 mL |
| 50 mM | 0.0689 mL | 0.3443 mL | 0.6886 mL | 1.3772 mL | 1.7215 mL |
| 100 mM | 0.0344 mL | 0.1722 mL | 0.3443 mL | 0.6886 mL | 0.8608 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CGP 13501 is a positive allosteric modulator of GABAB receptor [1].
The GABAB receptor is a metabotropic transmembrane receptors for GABA that is linked via G-protein to potassium channel. It stimulates the opening of K+ channel and hyperpolarize the neuron.
CGP 13501 is a positive allosteric modulator of GABAB receptor. In Chinese hamster ovary (CHO) cell line expressing GABAB(1b/2), CGP 13501 potentiated GABA-stimulated GTPγ[35S] binding to cell membranes [1]. In rat cortex, CGP13501 (10 μM) enhanced GABA-mediated activation of Gαo and increased GTPγ[35S] binding in the presence of GABA. Also, in the hippocampus and cerebellum, CGP13501 increased GABA-induced GTPγ[35S] binding and activation of Gαo. In rat cortex, hippocampus and cerebellum, CGP55845A blocked the influence of CGP13501 on GABA-induced GTPγ[35S] binding at Gαo with pIC50 values of 7.4, 7.31 and 6.82, respectively. In cells expressing GABAB(1b+2) receptors, CGP13501 increased GTPγ[35S] binding at Gαi1/3 [2].
References:
[1]. Urwyler S, Mosbacher J, Lingenhoehl K, et al. Positive allosteric modulation of native and recombinant gamma-aminobutyric acid(B) receptors by 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Mol Pharmacol, 2001, 60(5): 963-971.
[2]. Rasheed N, Ahmad A, Al-Sheeha M, et al. Neuroprotective and anti-stress effect of A68930 in acute and chronic unpredictable stress model in rats. Neurosci Lett, 2011, 504(2): 151-155.
- Acarbose
Catalog No.:BCC1190
CAS No.:56180-94-0
- H-Sar-OtBu.HCl
Catalog No.:BCC3336
CAS No.:5616-81-9
- Valrubicin
Catalog No.:BCC5219
CAS No.:56124-62-0
- 4-O-Methylhelichrysetin
Catalog No.:BCN3986
CAS No.:56121-44-9
- Asperglaucide
Catalog No.:BCN5748
CAS No.:56121-42-7
- Parisaponin I
Catalog No.:BCN2835
CAS No.:561007-63-4
- Securinine
Catalog No.:BCN6988
CAS No.:5610-40-2
- Ionomycin calcium salt
Catalog No.:BCC5805
CAS No.:56092-82-1
- Ionomycin free acid
Catalog No.:BCC7261
CAS No.:56092-81-0
- 3-Methoxyshancigusin I
Catalog No.:BCC9002
CAS No.:
- PRIMA-1
Catalog No.:BCC2413
CAS No.:5608-24-2
- Hispidin
Catalog No.:BCN3567
CAS No.:56070-89-4
- Terpinine-4-ol
Catalog No.:BCN8250
CAS No.:562-74-3
- Torsemide
Catalog No.:BCC4871
CAS No.:56211-40-6
- Methyl lycernuate A
Catalog No.:BCN5749
CAS No.:56218-46-3
- Porson
Catalog No.:BCN5750
CAS No.:56222-03-8
- Cyclo(Tyr-Gly)
Catalog No.:BCN2414
CAS No.:5625-49-0
- Cyclo(Leu-Val)
Catalog No.:BCN2436
CAS No.:5625-50-3
- Hydroxyanigorufone
Catalog No.:BCN7184
CAS No.:56252-02-9
- 2-O-Methylanigorufone
Catalog No.:BCN7180
CAS No.:56252-05-2
- Anigorufone
Catalog No.:BCN7171
CAS No.:56252-32-5
- Uncarine C
Catalog No.:BCC8261
CAS No.:5629-60-7
- Aloperine
Catalog No.:BCN8466
CAS No.:56293-29-9
- Fluoxetine HCl
Catalog No.:BCC1191
CAS No.:56296-78-7
Effect of GABAB receptor antagonists (CGP 35348 and CGP 55845) on serum interleukin 6 and 18 concentrations in albino mice following neonatal hypoxia ischemia insult.[Pubmed:27731803]
Pak J Pharm Sci. 2016 Sep;29(5):1503-1508.
Interleukin (IL) 6 and 18 plays an important role in inflammatory response following hypoxia ischemia encephalopathy (HIE). Present study was designed to demonstrate the effect of two GABAB receptor antagonists (CGP 35348 and 55845), respectively, on the serum IL6 and IL 18 concentrations in albino mice. Albino mice pups (of both genders) were subjected to Murine model of hypoxia-ischemia encephalopathy on postnatal day 10 (right common carotid artery was ligated followed by 8% hypoxia for 25 minutes). After neonatal brain damage and following weaning, mice were divided in three groups, in gender specific manner, and fed on normal rodent diet till they were 13 week old. At this time point, group 1 received intraperitonial saline solution (control group), group 2 was supplemented with CGP 35348 (1mg/ml solvent/Kg body weight) and group 3 with CGP 55845 (1mg/ml solvent/Kg body weight), intraperitonially, for 12 days and IL 6 and 18 concentrations were determined in serum by ELISA. It was observed that CGP 35348 supplementation resulted in reduced interlukin-6 and interlukin-18 concentrations in male albino mice. While CGP 55845 supplementation increased IL-6 and IL-18 concentrations in female albino mice following HIE. Our results are indicating that GABAB receptor antagonist's supplementation affects IL concentrations in albino mice in a gender specific manner following neonatal brain damage and can be further explored for the treatments of hypoxia ischemia associated neurological ailments.
Comprehensive genomic profiling (CGP) of ovarian clear cell carcinomas (OCCC) identifies clinically relevant genomic alterations (CRGA) and targeted therapy options.[Pubmed:28349114]
Gynecol Oncol Rep. 2017 Mar 1;20:62-66.
*MTOR pathway genes are often mutated in ovarian clear cell carcinomas (OCCC).*11.2% of OCCC have targetable alterations only in the mTOR pathway.*MTOR pathway mutations in OCCC can underlie robust, lasting responses to everolimus.
Gender-specific effects of CGP 55845, GABAB receptor antagonist, on neuromuscular coordination, learning and memory formation in albino mouse following neonatal hypoxia-ischemia insult.[Pubmed:25847084]
Neurol Sci. 2015 Jun;36(6):961-9.
GABAB receptor antagonists are experimentally proved as spatial memory enhancers in mouse models but their role has not been described following hypoxic-ischemic insult. 10-day-old albino mice were subjected to Murine model of hypoxia and ischemia. Following brain damage, mice were fed on normal rodent diet till they were 13 weeks old. At this time point, mice were divided into two groups. Group 1 received saline and group 2 received intraperitoneally CGP 55845 (1 mg/ml solvent/Kg body weight) for 12 days. Behavioural observations were made during rota rod, open field and Morris water maze test along with brain infarct measurement in both CGP 55845 treated and untreated groups. It was observed that application of GABAB receptor antagonist improved the over all motor function in male and female albino mice but effects were more pronounced in males. In open field, CGP 55845-treated female mice showed poor performance. CGP 55845 had no significant effect on learning and memory formation during Morris water maze test and also on brain infract size in both genders following hypoxia ischemia encephalopathy. Effects of CGP 55845 can be further explored in a dose and duration dependent manner to improve the learning and memory in albino mice following neonatal brain damage.
Positive allosteric modulation of native and recombinant gamma-aminobutyric acid(B) receptors by 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501.[Pubmed:11641424]
Mol Pharmacol. 2001 Nov;60(5):963-71.
The compounds CGP7930 [2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol] and its close analog CGP13501 were identified as positive modulators of gamma-aminobutyric acid(B) (GABA(B)) receptor function. They potentiate GABA-stimulated guanosine 5'-O-(3-[(35)S]thiotriphosphate) (GTP gamma[(35)S]) binding to membranes from a GABA(B(1b/2)) expressing Chinese hamster ovary (CHO) cell line at low micromolar concentrations and are ineffective in the absence of GABA. The structurally related compounds propofol and malonoben are inactive. Similar effects of CGP7930 are seen in a GTP gamma[(35)S] binding assay using a native GABA(B) receptor preparation (rat brain membranes). Receptor selectivity is demonstrated because no modulation of glutamate-induced GTP gamma[(35)S] binding is seen in a CHO cell line expressing the metabotropic glutamate receptor subtype 2. Dose-response curves with GABA in the presence of different fixed concentrations of CGP7930 reveal an increase of both the potency and maximal efficacy of GABA at the GABA(B(1b/2)) heteromer. Radioligand binding studies show that CGP7930 increases the affinity of agonists but acts at a site different from the agonist binding site. Agonist affinity is not modulated by CGP7930 at homomeric GABA(B(1b)) receptors. In addition to GTP gamma[(35)S] binding, we show that CGP7930 also has modulatory effects in cellular assays such as GABA(B) receptor-mediated activation of inwardly rectifying potassium channels in Xenopus laevis oocytes and Ca(2+) signaling in human embryonic kidney 293 cells. Furthermore, we show that CGP7930 enhances the inhibitory effect of L-baclofen on the oscillatory activity of cultured cortical neurons. This first demonstration of positive allosteric modulation at GABA(B) receptors may represent a novel means of therapeutic interference with the GABA-ergic system.


