Parisaponin ICAS# 561007-63-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 561007-63-4 | SDF | Download SDF |
| PubChem ID | 102004850 | Appearance | Powder |
| Formula | C50H82O22 | M.Wt | 1035.2 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
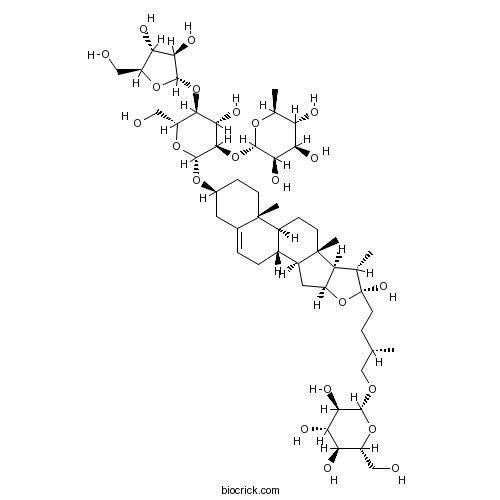
| SMILES | CC1C2C(CC3C2(CCC4C3CC=C5C4(CCC(C5)OC6C(C(C(C(O6)CO)OC7C(C(C(O7)CO)O)O)O)OC8C(C(C(C(O8)C)O)O)O)C)C)OC1(CCC(C)COC9C(C(C(C(O9)CO)O)O)O)O | ||
| Standard InChIKey | FAFUWLJMHOPRJO-SQHIPJLNSA-N | ||
| Standard InChI | InChI=1S/C50H82O22/c1-20(19-64-44-39(60)37(58)34(55)29(16-51)67-44)8-13-50(63)21(2)32-28(72-50)15-27-25-7-6-23-14-24(9-11-48(23,4)26(25)10-12-49(27,32)5)66-47-43(71-45-40(61)36(57)33(54)22(3)65-45)41(62)42(31(18-53)69-47)70-46-38(59)35(56)30(17-52)68-46/h6,20-22,24-47,51-63H,7-19H2,1-5H3/t20-,21-,22-,24-,25+,26-,27-,28-,29+,30-,31+,32-,33-,34+,35-,36+,37-,38+,39+,40+,41-,42+,43+,44+,45-,46-,47+,48-,49-,50+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Parisaponin I is a natural product from Paris yunnanensis Franch. |
| Structure Identification | 《Journal of Jinan University(Natural Science & Medicine Edition)》 2014-01Chemical constituents of Paris polyphylla var. yunnanensis[Reference: WebLink]The chemical constituents of Paris polyphylla var. yunnanensis were isolated by several chromatographic methods,including silica gel,Sephadex LH-20,ODS CC and preparation HPLC.
Bioorg Med Chem Lett. 2003 Mar 24;13(6):1101-6.Protective effects of steroid saponins from Paris polyphylla var. yunnanensis on ethanol- or indomethacin-induced gastric mucosal lesions in rats: structural requirement for activity and mode of action.[Reference: WebLink]
|

Parisaponin I Dilution Calculator

Parisaponin I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.966 mL | 4.83 mL | 9.66 mL | 19.3199 mL | 24.1499 mL |
| 5 mM | 0.1932 mL | 0.966 mL | 1.932 mL | 3.864 mL | 4.83 mL |
| 10 mM | 0.0966 mL | 0.483 mL | 0.966 mL | 1.932 mL | 2.415 mL |
| 50 mM | 0.0193 mL | 0.0966 mL | 0.1932 mL | 0.3864 mL | 0.483 mL |
| 100 mM | 0.0097 mL | 0.0483 mL | 0.0966 mL | 0.1932 mL | 0.2415 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Securinine
Catalog No.:BCN6988
CAS No.:5610-40-2
- Ionomycin calcium salt
Catalog No.:BCC5805
CAS No.:56092-82-1
- Ionomycin free acid
Catalog No.:BCC7261
CAS No.:56092-81-0
- 3-Methoxyshancigusin I
Catalog No.:BCC9002
CAS No.:
- PRIMA-1
Catalog No.:BCC2413
CAS No.:5608-24-2
- Hispidin
Catalog No.:BCN3567
CAS No.:56070-89-4
- Sucralose
Catalog No.:BCC4725
CAS No.:56038-13-2
- Eburicoic acid
Catalog No.:BCN2556
CAS No.:560-66-7
- 9-Hydroxy-4-androstene-3,17-dione
Catalog No.:BCC8802
CAS No.:560-62-3
- Chlorhexidine acetate
Catalog No.:BCC8912
CAS No.:56-95-1
- Histamine 2HCl
Catalog No.:BCC4530
CAS No.:56-92-8
- (H-Cys-OH)2
Catalog No.:BCC2915
CAS No.:56-89-3
- Asperglaucide
Catalog No.:BCN5748
CAS No.:56121-42-7
- 4-O-Methylhelichrysetin
Catalog No.:BCN3986
CAS No.:56121-44-9
- Valrubicin
Catalog No.:BCC5219
CAS No.:56124-62-0
- H-Sar-OtBu.HCl
Catalog No.:BCC3336
CAS No.:5616-81-9
- Acarbose
Catalog No.:BCC1190
CAS No.:56180-94-0
- CGP 13501
Catalog No.:BCC7097
CAS No.:56189-68-5
- Terpinine-4-ol
Catalog No.:BCN8250
CAS No.:562-74-3
- Torsemide
Catalog No.:BCC4871
CAS No.:56211-40-6
- Methyl lycernuate A
Catalog No.:BCN5749
CAS No.:56218-46-3
- Porson
Catalog No.:BCN5750
CAS No.:56222-03-8
- Cyclo(Tyr-Gly)
Catalog No.:BCN2414
CAS No.:5625-49-0
- Cyclo(Leu-Val)
Catalog No.:BCN2436
CAS No.:5625-50-3
Synthesis of furostanol glycosides: discovery of a potent alpha-glucosidase inhibitor.[Pubmed:27714262]
Org Biomol Chem. 2016 Oct 4;14(39):9362-9374.
A convenient approach to the synthesis of furostanol glycosides has been developed with the features of both highly efficient incorporation of a 26-O-beta-d-glucopyranosyl unit and ready formation of hemiketal ring E. The total syntheses of seven furostanol saponins including funlioside B, lilioglycoside, protobioside I, protodioscin, pallidifloside I, coreajaponins A and Parisaponin I are efficiently achieved using an easily available 16beta-acetoxy-22-oxo-26-hydroxy-cholestanic derivative as a powerful building block. The alpha-glucosidase inhibitory activity of the synthesized saponins is also evaluated, which reveals that funlioside B is a highly potential lead for developing alpha-glucosidase inhibitors.
New furostanol saponins from Allium ascalonicum L.[Pubmed:17661431]
Magn Reson Chem. 2007 Sep;45(9):725-33.
An analysis of the polar extracts from Allium ascalonicum L. led to the isolation of two new furostanol saponins (compound 1 and 2) and two known furostanol saponins (compound 3 and 4). On the basis of 1D and 2D NMR (including (1)H, (13)C NMR, (1)H--(1)H COSY, HSQC, TOCSY, HMBC, and NOESY), FAB-MS spectrometry, and chemical methods, their structures were elucidated as (25R)-26-O-beta-D-glucopyranosyl-22-hydroxy-5alpha-furost-2-one-3beta, 5, 6beta, 26-tetraol-3-O-alpha-L-rhamnopyranosyl-(1-->2)-beta-D-glucopyranoside (ascalonicoside C, 1), (25R)-26-O-beta-D-glucopyranosyl-22-methoxy-5alpha-furost-2-one-3beta, 5, 6beta, 26-tetraol- 3-O-alpha-L-rhamnopyranosyl-(1-->2)-beta-D-glucopyranoside (ascalonicoside D, 2), (25R)-26-O-beta-D-glucopyranosyl-22-hydroxy-5-ene-furostan-3beta, 26-diol-3-O-alpha-L-rhamnopyranosyl-(1-->4)-alpha-L-rhamnopyranosyl-(1-->4)-[alph a-L-rhamnopyranosyl-(1-->2)]-beta-D-glucopyranoside (dichotomin, 3), and (25R)-26-O-beta-D-glucopyranosyl-22-hydroxy-5-ene-furostan-3beta, 26-diol-3-O-alpha-L-rhamnopyranosyl-(1-->2)-[alpha-L-arabinofuranosyl-(1-->4)]-be ta-D- glucopyranoside (Parisaponin I, 4).
Protective effects of steroid saponins from Paris polyphylla var. yunnanensis on ethanol- or indomethacin-induced gastric mucosal lesions in rats: structural requirement for activity and mode of action.[Pubmed:12643921]
Bioorg Med Chem Lett. 2003 Mar 24;13(6):1101-6.
The methanolic extract from the rhizomes of Paris polyphylla SM. var. yunnanensis (FR.) H-M. was found to potently inhibit ethanol-induced gastric lesions in rats. Through bioassay-guided separation, four known spirostanol-type steroid saponins, pennogenin 3-O-alpha-L-rhamnopyranosyl(1-->2)-[alpha-L-arabinofuranosyl(1-->4)]-beta-D-gluco pyranoside (1), pennogenin 3-O-alpha-L-rhamnopyranosyl(1-->4)-alpha-L-rhamnopyranosyl(1-->4)-[alpha-L-rhamno pyranosyl(1-->2)]-beta-D-glucopyranoside (2), diosgenin 3-O-alpha-L-rhamnopyranosyl(1-->2)-[alpha-L-arabinofuranosyl(1-->4)]-beta-D-gluco pyranoside (3), and diosgenin 3-O-alpha-L-rhamnopyranosyl(1-->4)-alpha-L-rhamnopyranosyl(1-->4)-[alpha-L-rhamno pyranosyl(1-->2)]-beta-D-glucopyranoside (4), and a new furostanol-type steroid saponin, Parisaponin I (5), together with two known furostanol-type steroid saponins, trigofoenoside A (6) and protogracillin (7), were isolated from the active fraction. Compounds 1-4 (1.25-10 mg/kg, po) strongly inhibited gastric lesions induced by ethanol and indomethacin. With regard to structural requirement of steroid saponins, the 3-O-glycoside moiety and spirostanol structure were found to be essential for the activity and the 17-hydroxyl group in the aglycon part enhanced the protective effects against ethanol-induced gastric lesions. The protective effects of 1 and 3 against ethanol-induced gastric lesions were attenuated by pretreatment with indomethacin and N-ethylmaleimide. Compounds 1 and 3 weakly inhibited acid secretions in pylorus-ligated rats. These findings suggested that endogenous prostaglandins and sulfhydryl compounds were involved in the protective activity.


