IsomitraphyllineCAS# 4963-01-3 |
- Uncarine D
Catalog No.:BCC8262
CAS No.:4697-68-1
- Mitraphylline
Catalog No.:BCC8213
CAS No.:509-80-8
- Uncarine E
Catalog No.:BCC8263
CAS No.:5171-37-9
- Uncarine C
Catalog No.:BCC8261
CAS No.:5629-60-7
- Uncarine A
Catalog No.:BCN7767
CAS No.:6899-73-6
- Uncarine F
Catalog No.:BCN9446
CAS No.:14019-66-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4963-01-3 | SDF | Download SDF |
| PubChem ID | 11726520 | Appearance | White powder |
| Formula | C21H24N2O4 | M.Wt | 368.42 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Ajmalicine oxindole A | ||
| Solubility | Soluble in chloroform, diethyl ether and methanol; sparingly soluble in water | ||
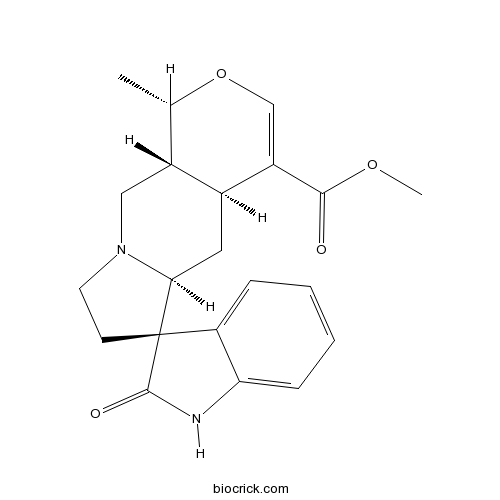
| Chemical Name | methyl (1S,4aS,5aS,6S,10aR)-1-methyl-2'-oxospiro[1,4a,5,5a,7,8,10,10a-octahydropyrano[3,4-f]indolizine-6,3'-1H-indole]-4-carboxylate | ||
| SMILES | CC1C2CN3CCC4(C3CC2C(=CO1)C(=O)OC)C5=CC=CC=C5NC4=O | ||
| Standard InChIKey | JMIAZDVHNCCPDM-NWQITLLVSA-N | ||
| Standard InChI | InChI=1S/C21H24N2O4/c1-12-14-10-23-8-7-21(16-5-3-4-6-17(16)22-20(21)25)18(23)9-13(14)15(11-27-12)19(24)26-2/h3-6,11-14,18H,7-10H2,1-2H3,(H,22,25)/t12-,13-,14+,18-,21-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Isomitraphylline and pteropodine are the most suitable for standardization of medical cat's claw preparations. 2. Isomitraphylline can inhibit proliferation of acute lymphoblastic leukaemia cells. |
| Targets | Bcl-2/Bax |

Isomitraphylline Dilution Calculator

Isomitraphylline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7143 mL | 13.5715 mL | 27.1429 mL | 54.2859 mL | 67.8573 mL |
| 5 mM | 0.5429 mL | 2.7143 mL | 5.4286 mL | 10.8572 mL | 13.5715 mL |
| 10 mM | 0.2714 mL | 1.3571 mL | 2.7143 mL | 5.4286 mL | 6.7857 mL |
| 50 mM | 0.0543 mL | 0.2714 mL | 0.5429 mL | 1.0857 mL | 1.3571 mL |
| 100 mM | 0.0271 mL | 0.1357 mL | 0.2714 mL | 0.5429 mL | 0.6786 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Angelicain
Catalog No.:BCN5605
CAS No.:49624-66-0
- Robustaflavone
Catalog No.:BCN8285
CAS No.:49620-13-5
- Tetraethylenepentamine 5HCl
Catalog No.:BCC3867
CAS No.:4961-41-5
- Helicianeoide B
Catalog No.:BCN2487
CAS No.:496066-89-8
- Helicianeoide A
Catalog No.:BCN2486
CAS No.:496066-82-1
- Pyromeconic acid
Catalog No.:BCN7177
CAS No.:496-63-9
- Benzofuran-2-carboxylic acid
Catalog No.:BCC8851
CAS No.:496-41-3
- Fenofibrate
Catalog No.:BCC4781
CAS No.:49562-28-9
- Estradiol heptanoate
Catalog No.:BCC8961
CAS No.:4956-37-0
- 11alpha,12alpha-Oxidotaraxerol palmitate
Catalog No.:BCN7129
CAS No.:495389-95-2
- Org 25543 hydrochloride
Catalog No.:BCC6288
CAS No.:495076-64-7
- (+)-Methysticin
Catalog No.:BCN8429
CAS No.:495-85-2
- Simiarenol acetate
Catalog No.:BCN5606
CAS No.:4965-99-5
- ZLN005
Catalog No.:BCC4882
CAS No.:49671-76-3
- Eltrombopag
Catalog No.:BCC4968
CAS No.:496775-61-2
- Eltrombopag Olamine
Catalog No.:BCC1549
CAS No.:496775-62-3
- Crobarbatine
Catalog No.:BCN2069
CAS No.:49679-23-4
- AR-C155858
Catalog No.:BCC1367
CAS No.:496791-37-8
- HhAntag
Catalog No.:BCC1617
CAS No.:496794-70-8
- Drupacine
Catalog No.:BCN7065
CAS No.:49686-57-9
- Adarotene
Catalog No.:BCC1328
CAS No.:496868-77-0
- Arbutin
Catalog No.:BCN6307
CAS No.:497-76-7
- (-)-Praeruptorin B
Catalog No.:BCN7665
CAS No.:4970-26-7
- Z-Thr-NH2
Catalog No.:BCC2738
CAS No.:49705-98-8
Long-term response on growth, antioxidant enzymes, and secondary metabolites in salicylic acid pre-treated Uncaria tomentosa microplants.[Pubmed:26272395]
Biotechnol Lett. 2015 Dec;37(12):2489-96.
OBJECTIVE: To obtain micro propagated Uncaria tomentosa plantlets with enhanced secondary metabolites production, long-term responses to salicylic acid (SA) pre-treatments at 1 and 100 microM were evaluated after propagation of the plantlets in a SA-free medium. RESULTS: SA pre-treatments of single node cuttings OF U. tomentosa produced long-term responses in microplants grown for 75 days in a SA-free medium. Reduction in survival rate, root formation, and stem elongation were observed only with 100 microM SA pre-treatments with respect to the control (0 + DMSO).Both pre-treatments enhanced H2O2 and inhibited superoxide dismutase and catalase activities, while guaiacol peroxidase was increased only with 1 microM SA. Also, both pre-treatments increased total monoterpenoid oxindole alkaloids by ca. 55 % (16.5 mg g(-1) DW), including isopteropodine, speciophylline, mitraphylline, Isomitraphylline, rhynchopylline, and isorhynchopylline; and flavonoids by ca. 21 % (914 mug g(-1) DW), whereas phenolic compounds were increased 80 % (599 mug g(-1) DW) at 1 microM and 8.2 % (359 mug g(-1) DW) at 100 microM SA. CONCLUSION: Pre-treatment with 1 microM SA of U.tomentosa microplants preserved the survival rate and increased oxindole alkaloids, flavonoids, and phenolic compounds in correlation with H2O2 and peroxidase activity enhancements, offering biotechnological advantages over non-treated microplants.
Oxindole alkaloids from Uncaria tomentosa induce apoptosis in proliferating, G0/G1-arrested and bcl-2-expressing acute lymphoblastic leukaemia cells.[Pubmed:16445836]
Br J Haematol. 2006 Mar;132(5):615-22.
Natural products are still an untapped source of promising lead compounds for the generation of antineoplastic drugs. Here, we investigated for the first time the antiproliferative and apoptotic effects of highly purified oxindole alkaloids, namely isopteropodine (A1), pteropodine (A2), Isomitraphylline (A3), uncarine F (A4) and mitraphylline (A5) obtained from Uncaria tomentosa, a South American Rubiaceae, on human lymphoblastic leukaemia T cells (CCRF-CEM-C7H2). Four of the five tested alkaloids inhibited proliferation of acute lymphoblastic leukaemia cells. Furthermore, the antiproliferative effect of the most potent alkaloids pteropodine (A2) and uncarine F (A4) correlated with induction of apoptosis. After 48 h, 100 micromol/l A2 or A4 increased apoptotic cells by 57%. CEM-C7H2 sublines with tetracycline-regulated expression of bcl-2, p16ink4A or constitutively expressing the cowpox virus protein crm-A were used for further studies of the apoptosis-inducing properties of these alkaloids. Neither overexpression of bcl-2 or crm-A nor cell-cycle arrest in G0/G1 phase by tetracycline-regulated expression of p16INK4A could prevent alkaloid-induced apoptosis. Our results show the strong apoptotic effects of pteropodine and uncarine F on acute leukaemic lymphoblasts and recommend the alkaloids for further studies in xenograft models.
Phytochemical characterization of the leaves of Mitragyna speciosa grown in U.S.A.[Pubmed:19731590]
Nat Prod Commun. 2009 Jul;4(7):907-10.
Mitragyna speciosa (Rubiaceae) has traditionally been used in the tropical regions of Asia, Africa and Indonesia as a substitute for opium. Indole alkaloids are the most common compounds that have been isolated. We investigated the constituents of the leaves of M. speciosa that was grown at the University of Mississippi. Several alkaloids were isolated, including ajmalicine, corynantheidine, Isomitraphylline, mitraphylline, paynantheine, isocorynantheidine, 7-hydroxymitragynine and mitragynine, but their percentages were lower than those in a commercial Thai sample of "kratom". In addition, we isolated the flavonoid epicatechin, a saponin daucosterol, the triterpenoid saponins quinovic acid 3-O-beta-D-quinovopyranoside, quinovic acid 3-O-beta-D-glucopyranoside, as well as several glycoside derivatives including 1-O-feruloyl-beta-D-glucopyranoside, benzyl-beta-D-glucopyranoside, 3-oxo-alpha-ionyl-O-beta-D-glucopyranoside, roseoside, vogeloside, and epivogeloside. This is the first report of the last group of compounds having been isolated from a Mitragyna species. Biological studies are currently underway to test these compounds for opioid activity.
Antiproliferative activity of various Uncaria tomentosa preparations on HL-60 promyelocytic leukemia cells.[Pubmed:18048957]
Pharmacol Rep. 2007 Sep-Oct;59(5):565-72.
The woody Amazonian vine Uncaria tomentosa (cat's claw) has been recently more and more popular all over the world as an immunomodulatory, antiinflammatory and anti-cancer remedy. This study investigates anti-proliferative potency of several cat's claw preparations with different quantitative and qualitative alkaloid contents on HL-60 acute promyelocytic human cells by applying trypan blue exclusion and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reduction assay (MTT). By standardization and statistical comparison of the obtained results pteropodine and Isomitraphylline are indicated to be most suitable for standardization of medical cat's claw preparations.


