EltrombopagCAS# 496775-61-2 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 496775-61-2 | SDF | Download SDF |
| PubChem ID | 9846180 | Appearance | Powder |
| Formula | C25H22N4O4 | M.Wt | 442.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SB-497115; SB-497115-GR | ||
| Solubility | Soluble in DMSO > 10 mM | ||
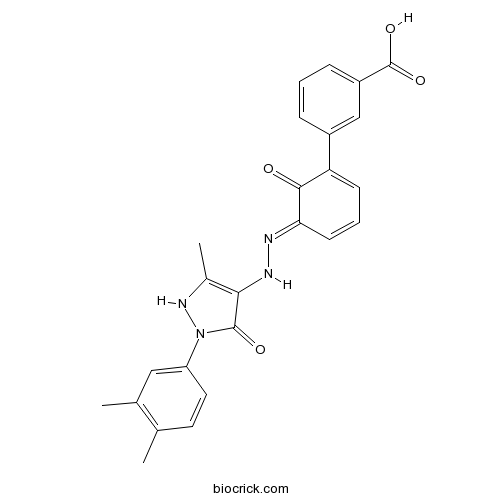
| Chemical Name | 3-[(5E)-5-[[2-(3,4-dimethylphenyl)-5-methyl-3-oxo-1H-pyrazol-4-yl]hydrazinylidene]-6-oxocyclohexa-1,3-dien-1-yl]benzoic acid | ||
| SMILES | CC1=C(C=C(C=C1)N2C(=O)C(=C(N2)C)NN=C3C=CC=C(C3=O)C4=CC(=CC=C4)C(=O)O)C | ||
| Standard InChIKey | TYEXNVNUZXJNBN-YYADALCUSA-N | ||
| Standard InChI | InChI=1S/C25H22N4O4/c1-14-10-11-19(12-15(14)2)29-24(31)22(16(3)28-29)27-26-21-9-5-8-20(23(21)30)17-6-4-7-18(13-17)25(32)33/h4-13,27-28H,1-3H3,(H,32,33)/b26-21+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Eltrombopag(SB-497115) is a new, orally active thrombopoietin-receptor (c-mpl) agonist that stimulates thrombopoiesis.
IC50 Value: 0.27 uM (EC50 in murine BAF3 cells) [1]
Target: thrombopoietin-receptor (c-mpl)
Potential advantages of eltrombopag may include a sustained platelet response and a good tolerability profile.
in vitro: Eltrombopag demonstrated a half maximal effective concentration (EC50) of 0.27 uM in murine BAF3 cells transfected with the luciferase reporter gene under direction of the STAT-activated IRF-1 promoter and human TpoR (BAF3/IRF-1/hTpoR) [1]. Eltrombopag stimulates the growth of TPO-dependent cell lines via JAK2 and STAT signaling pathways and stimulates isolated human CD34+ cells to become megakaryocytes and produce platelets [2].
in vivo: Twelve weeks of antiviral therapy, with concurrent receipt of eltrombopag or placebo, were completed by 36%, 53%, and 65% of patients receiving 30 mg, 50 mg, and 75 mg of eltrombopag, respectively, and by 6% of patients in the placebo group [3]. Eltrombopag was administered as once-daily oral capsules for 10 days at doses of 5, 10, 25, 30, 50, and 75 mg. The pharmacokinetics of eltrombopag was dose dependent and linear, and eltrombopag increased platelet counts in a dose-dependent manner [4].
Toxicity: There were no apparent differences in the incidence or severity of adverse events in subjects receiving active or placebo study medication [4]. Long-term treatment with eltrombopag was generally safe, well tolerated, and effective in maintaining platelet counts in the desired range [5].
Clinical trial: Eltrombopag In Cord Blood Or Haploidentical Bone Marrow Transplantation. Phase 2 References: | |||||

Eltrombopag Dilution Calculator

Eltrombopag Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.26 mL | 11.3002 mL | 22.6004 mL | 45.2008 mL | 56.501 mL |
| 5 mM | 0.452 mL | 2.26 mL | 4.5201 mL | 9.0402 mL | 11.3002 mL |
| 10 mM | 0.226 mL | 1.13 mL | 2.26 mL | 4.5201 mL | 5.6501 mL |
| 50 mM | 0.0452 mL | 0.226 mL | 0.452 mL | 0.904 mL | 1.13 mL |
| 100 mM | 0.0226 mL | 0.113 mL | 0.226 mL | 0.452 mL | 0.565 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Eltrombopag is a new, orally active thrombopoietin-receptor (c-mpl) agonist that stimulates thrombopoiesis.
- ZLN005
Catalog No.:BCC4882
CAS No.:49671-76-3
- Simiarenol acetate
Catalog No.:BCN5606
CAS No.:4965-99-5
- Isomitraphylline
Catalog No.:BCN7800
CAS No.:4963-01-3
- Angelicain
Catalog No.:BCN5605
CAS No.:49624-66-0
- Robustaflavone
Catalog No.:BCN8285
CAS No.:49620-13-5
- Tetraethylenepentamine 5HCl
Catalog No.:BCC3867
CAS No.:4961-41-5
- Helicianeoide B
Catalog No.:BCN2487
CAS No.:496066-89-8
- Helicianeoide A
Catalog No.:BCN2486
CAS No.:496066-82-1
- Pyromeconic acid
Catalog No.:BCN7177
CAS No.:496-63-9
- Benzofuran-2-carboxylic acid
Catalog No.:BCC8851
CAS No.:496-41-3
- Fenofibrate
Catalog No.:BCC4781
CAS No.:49562-28-9
- Estradiol heptanoate
Catalog No.:BCC8961
CAS No.:4956-37-0
- Eltrombopag Olamine
Catalog No.:BCC1549
CAS No.:496775-62-3
- Crobarbatine
Catalog No.:BCN2069
CAS No.:49679-23-4
- AR-C155858
Catalog No.:BCC1367
CAS No.:496791-37-8
- HhAntag
Catalog No.:BCC1617
CAS No.:496794-70-8
- Drupacine
Catalog No.:BCN7065
CAS No.:49686-57-9
- Adarotene
Catalog No.:BCC1328
CAS No.:496868-77-0
- Arbutin
Catalog No.:BCN6307
CAS No.:497-76-7
- (-)-Praeruptorin B
Catalog No.:BCN7665
CAS No.:4970-26-7
- Z-Thr-NH2
Catalog No.:BCC2738
CAS No.:49705-98-8
- DC_AC50
Catalog No.:BCC6488
CAS No.:497061-48-0
- Tupichinol A
Catalog No.:BCN7697
CAS No.:497142-88-8
- Dobutamine hydrochloride
Catalog No.:BCC5391
CAS No.:49745-95-1
[Eltrombopag for refractory thrombocytopenia in patients with allogeneic hematopoietic stem cell transplantation].[Pubmed:28088971]
Zhonghua Xue Ye Xue Za Zhi. 2016 Dec 14;37(12):1065-1069.
Objective: To evaluate the efficacy and safety of Eltrombopag in post-HSCT thrombocytopenia. Methods: A total of 10 patients who underwent post-HSCT thrombocytopenia at Peking University center, who had been treated with Eltrombopag, were retrospectively evaluated. Results: Of the 10 cases, 5 males and 5 females with a median of 34 years old (range, 17-54 years), 5 patients were acute myeloid leukemia, 3 with acute lymphoid leukemia and 2 with severe aplastic anemia. Nine patients had undergone haplo-identical donor transplantation, and one patient was a matched related recipient. All patients had failed prior treatment for thrombocytopenia before Eltrombopag started. The median time when Eltrombopag started was 221 days (range, 73-917 days) after transplantation. Five patients (50%) had achieved CR. The cumulative incidence of 30-day CR was 35.7%. The median time to platelet recovery >/= 50 x 10(9)/L without transfusion support was 16 days (range, 10-56 days). At the last follow-up, three of the patients with CR had withdrawal Eltrombopag and remained normal platelet counts. No patients experienced drug-related adverse events. Conclusion: Eltrombopag is effective and well tolerated in patients with refractory post-HSCT thrombocytopenia.
Eltrombopag versus placebo for low-risk myelodysplastic syndromes with thrombocytopenia (EQoL-MDS): phase 1 results of a single-blind, randomised, controlled, phase 2 superiority trial.[Pubmed:28162984]
Lancet Haematol. 2017 Mar;4(3):e127-e136.
BACKGROUND: In myelodysplastic syndromes, thrombocytopenia is associated with mortality, but treatments in this setting are scarce. We tested whether Eltrombopag, a thrombopoietin receptor agonist, might be effective in improving thrombocytopenia in lower-risk myelodysplastic syndromes and severe thrombocytopenia. METHODS: EQoL-MDS was a single-blind, randomised, controlled, phase 2 superiority trial of adult patients with low-risk or International Prognostic Scoring System intermediate-1-risk myelodysplastic syndromes and severe thrombocytopenia. Patients with a stable platelet count of lower than 30 x 10(9) platelets per L, aged at least 18 years, with refractoriness, ineligibility to receive treatment with alternative medications, or relapse while receiving treatment with alternative medications were included in this trial. Patients were randomly assigned (2:1) to receive Eltrombopag (50 mg to 300 mg) or placebo for at least 24 weeks and until disease progression and were masked to treatment allocation. Here, we report the results in the intention-to-treat population of the first phase of the trial, for which the primary endpoints were the proportion of patients achieving a platelet response within 24 weeks and safety. The interim analysis presented here was protocol-specified and used a two-sided significance level of 0.001 and a p value at or below this limit for both primary endpoints to indicate the need for early trial termination. Duration of platelet transfusion independence, duration of response, overall survival, leukaemia-free survival, and pharmacokinetics will be reported at the end of the phase 2 portion of the trial. This trial is registered with EudraCT, number 2010-022890-33. FINDINGS: Between June 13, 2011, and June 17, 2016, we enrolled 90 participants for the first phase of the trial. The median follow-up time to assess platelet responses was 11 weeks (IQR 4-24). Platelet responses occurred in 28 (47%) of 59 patients in the Eltrombopag group versus one (3%) of 31 patients in the placebo group (odds ratio 27.1 [95% CI 3.5-211.9], p=0.0017). During the follow-up, 21 patients had at least one severe bleeding event (WHO bleeding score >/=2). There were a higher number of bleeders in the placebo (13 [42%] of 31 patients) than in the Eltrombopag arm (eight [14%] of 59 patients; p=0.0025). 52 grade 3-4 adverse events occurred in 27 (46%) of 59 patients in the Eltrombopag group versus nine events in five (16%) of 31 patients in the placebo group (chi(2)=7.8, p=0.0053, stopping rule not reached). The outcome acute myeloid leukaemia evolution or disease progression occurred in seven (12%) of 59 patients in the Eltrombopag group versus five (16%) of 31 patients in the placebo group (chi(2)=0.06, p=0.81). INTERPRETATION: Eltrombopag is well-tolerated in patients with lower-risk myelodysplastic syndromes and severe thrombocytopenia and is clinically effective in raising platelet counts and reducing bleeding events. The assessment of long-term safety and efficacy of Eltrombopag and its effect on survival (phase 2 part of study) is still ongoing. FUNDING: Associazione QOL-ONE.


