AdaroteneApoptosis inducer/DNA damage agent CAS# 496868-77-0 |
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- DPH
Catalog No.:BCC1538
CAS No.:484049-04-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 496868-77-0 | SDF | Download SDF |
| PubChem ID | 9864378 | Appearance | Powder |
| Formula | C25H26O3 | M.Wt | 374.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ST1926 | ||
| Solubility | DMSO : 25 mg/mL (66.76 mM; Need ultrasonic) | ||
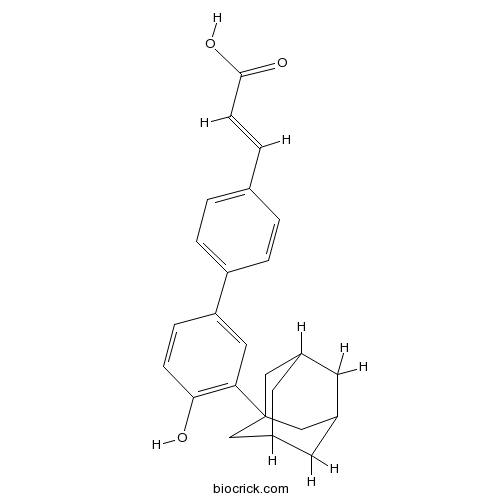
| Chemical Name | (E)-3-[4-[3-(1-adamantyl)-4-hydroxyphenyl]phenyl]prop-2-enoic acid | ||
| SMILES | C1C2CC3CC1CC(C2)(C3)C4=C(C=CC(=C4)C5=CC=C(C=C5)C=CC(=O)O)O | ||
| Standard InChIKey | QAWBIEIZDDIEMW-FPYGCLRLSA-N | ||
| Standard InChI | InChI=1S/C25H26O3/c26-23-7-6-21(20-4-1-16(2-5-20)3-8-24(27)28)12-22(23)25-13-17-9-18(14-25)11-19(10-17)15-25/h1-8,12,17-19,26H,9-11,13-15H2,(H,27,28)/b8-3+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Adarotene is a new-in-class potent proapoptotic and cytodifferentiating agent act as a selective activator of RAR β and RAR γ. | |||||
| Targets | RAR β | RAR γ | ||||

Adarotene Dilution Calculator

Adarotene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6704 mL | 13.3522 mL | 26.7044 mL | 53.4088 mL | 66.761 mL |
| 5 mM | 0.5341 mL | 2.6704 mL | 5.3409 mL | 10.6818 mL | 13.3522 mL |
| 10 mM | 0.267 mL | 1.3352 mL | 2.6704 mL | 5.3409 mL | 6.6761 mL |
| 50 mM | 0.0534 mL | 0.267 mL | 0.5341 mL | 1.0682 mL | 1.3352 mL |
| 100 mM | 0.0267 mL | 0.1335 mL | 0.267 mL | 0.5341 mL | 0.6676 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Retinoid-related molecules are derivatives of retinoic acid and promising antileukemic agents with a mechanism of action different from that of other common chemotherapeutics. Adarotene is a novel atypical retinoid endowed with proapoptotic and antitumor activity.
In vitro: The novel atypical retinoid adarotene exhibited potent antiproliferative activity on a large panel of human tumor cells. It was found that although almost complete loss of ability to activate RARs, adarotene was performing as an effective apoptosis inducer and surprisingly produced DNA damage, which likely contributed to its proapoptotic activity [1].
In vivo: Following oral administration, adarotene was well tolerated and caused tumor growth inhibition in the ovarian carcinoma (A2780/DX) and the human melanoma (MeWo) exnographt in nude mice, supporting the therapeutic interest of this novel agent [1].
Clinical trial: Adarotene is currently only in the preclinical developlent stage and no clinical data are available.
Reference:
[1] Cincinelli R, Dallavalle S, Merlini L, Penco S, Pisano C, Carminati P, Giannini G, Vesci L, Gaetano C, Illy B, Zuco V, Supino R, Zunino F. A novel atypical retinoid endowed with proapoptotic and antitumor activity. J Med Chem. 2003;46(6):909-12.
- Drupacine
Catalog No.:BCN7065
CAS No.:49686-57-9
- HhAntag
Catalog No.:BCC1617
CAS No.:496794-70-8
- AR-C155858
Catalog No.:BCC1367
CAS No.:496791-37-8
- Crobarbatine
Catalog No.:BCN2069
CAS No.:49679-23-4
- Eltrombopag Olamine
Catalog No.:BCC1549
CAS No.:496775-62-3
- Eltrombopag
Catalog No.:BCC4968
CAS No.:496775-61-2
- ZLN005
Catalog No.:BCC4882
CAS No.:49671-76-3
- Simiarenol acetate
Catalog No.:BCN5606
CAS No.:4965-99-5
- Isomitraphylline
Catalog No.:BCN7800
CAS No.:4963-01-3
- Angelicain
Catalog No.:BCN5605
CAS No.:49624-66-0
- Robustaflavone
Catalog No.:BCN8285
CAS No.:49620-13-5
- Tetraethylenepentamine 5HCl
Catalog No.:BCC3867
CAS No.:4961-41-5
- Arbutin
Catalog No.:BCN6307
CAS No.:497-76-7
- (-)-Praeruptorin B
Catalog No.:BCN7665
CAS No.:4970-26-7
- Z-Thr-NH2
Catalog No.:BCC2738
CAS No.:49705-98-8
- DC_AC50
Catalog No.:BCC6488
CAS No.:497061-48-0
- Tupichinol A
Catalog No.:BCN7697
CAS No.:497142-88-8
- Dobutamine hydrochloride
Catalog No.:BCC5391
CAS No.:49745-95-1
- H-D-Phe(4-Me)-OH
Catalog No.:BCC3271
CAS No.:49759-61-7
- Stiripentol
Catalog No.:BCC3977
CAS No.:49763-96-4
- 11beta-Hydroxylupeol
Catalog No.:BCN7571
CAS No.:49776-92-3
- AEE788 (NVP-AEE788)
Catalog No.:BCC2520
CAS No.:497839-62-0
- Vanillyl alcohol
Catalog No.:BCN3832
CAS No.:498-00-0
- Acetovanillone
Catalog No.:BCN2916
CAS No.:498-02-2
Stimulation of Erythrocyte Cell Membrane Scrambling by Adarotene.[Pubmed:28214860]
Cell Physiol Biochem. 2017;41(2):519-529.
BACKGROUND/AIMS: The atypical retinoid E23-(40-hydroxyl-30-adamantylbiphenyl-4-yl) acrylic acid (ST1926, Adarotene) is used in the treatment of malignancy. The effect of ST1926 is at least in part due to stimulation of apoptosis. Similar to apoptosis of nucleated cells, erythrocytes may enter eryptosis, the suicidal death of erythrocytes. Hallmarks of eryptosis include cell shrinkage and cell membrane scrambling with phosphatidylserine translocation to the erythrocyte surface. Signaling involved in the stimulation of eryptosis includes increase of cytosolic Ca2+ activity [Ca2+]i, oxidative stress and ceramide. The present study explored, whether Adarotene induces eryptosis and, if so, to test for the involvement of Ca2+ entry, oxidative stress and ceramide. METHODS: Flow cytometry was employed to estimate phosphatidylserine exposure at the cell surface from annexin-V-binding, cell volume from forward scatter, [Ca2+]i from Fluo3-fluorescence, reactive oxygen species (ROS) formation from DCFDA dependent fluorescence, and ceramide abundance utilizing specific antibodies. RESULTS: A 48 hours exposure of human erythrocytes to Adarotene (9 microM) significantly increased the percentage of annexin-V-binding cells, an effect paralleled by significant decrease of forward scatter, as well as significant increase of Fluo3-fluorescence, DCFDA fluorescence, and ceramide abundance. The effect of Adarotene (9 microM) on annexin-V-binding was significantly blunted but not abolished by removal of extracellular Ca2+. CONCLUSIONS: Adarotene stimulates phospholipid scrambling of the erythrocyte cell membrane, an effect paralleled by and at least in part due to Ca2+ entry, oxidative stress and ceramide.
New retinoid derivatives as back-ups of Adarotene.[Pubmed:22365912]
Bioorg Med Chem. 2012 Apr 1;20(7):2405-15.
Adarotene belongs to the so-called class of atypical retinoids. The presence of the phenolic hydroxyl group on Adarotene structure allows a rapid O-glucuronidation as a major mechanism of elimination of the drug, favoring a fast excretion of its glucuronide metabolite in the urines. A series of ether, carbamate and ester derivatives was synthesized. All of them were studied and evaluated for their stability at different pH. The cytotoxic activity in vitro on NCI-H460 non-small cell lung carcinoma and A2780 ovarian tumor cell lines was also tested. A potential back-up of Adarotene has been selected to be evaluated in tumor models.


