Dobutamine hydrochlorideCAS# 49745-95-1 |
- ML347
Catalog No.:BCC5331
CAS No.:1062368-49-3
- LDN-212854
Catalog No.:BCC5330
CAS No.:1432597-26-6
- PD 169316
Catalog No.:BCC3969
CAS No.:152121-53-4
- Imperatorin
Catalog No.:BCN5574
CAS No.:482-44-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 49745-95-1 | SDF | Download SDF |
| PubChem ID | 65324 | Appearance | Powder |
| Formula | C18H24ClNO3 | M.Wt | 337.84 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 33 mg/mL (97.68 mM) H2O : 20 mg/mL (59.20 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
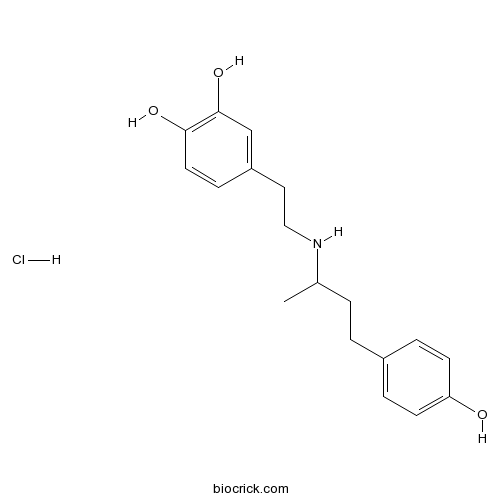
| Chemical Name | 4-[2-[4-(4-hydroxyphenyl)butan-2-ylamino]ethyl]benzene-1,2-diol;hydrochloride | ||
| SMILES | CC(CCC1=CC=C(C=C1)O)NCCC2=CC(=C(C=C2)O)O.Cl | ||
| Standard InChIKey | BQKADKWNRWCIJL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H23NO3.ClH/c1-13(2-3-14-4-7-16(20)8-5-14)19-11-10-15-6-9-17(21)18(22)12-15;/h4-9,12-13,19-22H,2-3,10-11H2,1H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | β1- and β2-adrenoceptor agonist with some action at the α1-adrenoceptor. |

Dobutamine hydrochloride Dilution Calculator

Dobutamine hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.96 mL | 14.7999 mL | 29.5998 mL | 59.1996 mL | 73.9995 mL |
| 5 mM | 0.592 mL | 2.96 mL | 5.92 mL | 11.8399 mL | 14.7999 mL |
| 10 mM | 0.296 mL | 1.48 mL | 2.96 mL | 5.92 mL | 7.4 mL |
| 50 mM | 0.0592 mL | 0.296 mL | 0.592 mL | 1.184 mL | 1.48 mL |
| 100 mM | 0.0296 mL | 0.148 mL | 0.296 mL | 0.592 mL | 0.74 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tupichinol A
Catalog No.:BCN7697
CAS No.:497142-88-8
- DC_AC50
Catalog No.:BCC6488
CAS No.:497061-48-0
- Z-Thr-NH2
Catalog No.:BCC2738
CAS No.:49705-98-8
- (-)-Praeruptorin B
Catalog No.:BCN7665
CAS No.:4970-26-7
- Arbutin
Catalog No.:BCN6307
CAS No.:497-76-7
- Adarotene
Catalog No.:BCC1328
CAS No.:496868-77-0
- Drupacine
Catalog No.:BCN7065
CAS No.:49686-57-9
- HhAntag
Catalog No.:BCC1617
CAS No.:496794-70-8
- AR-C155858
Catalog No.:BCC1367
CAS No.:496791-37-8
- Crobarbatine
Catalog No.:BCN2069
CAS No.:49679-23-4
- Eltrombopag Olamine
Catalog No.:BCC1549
CAS No.:496775-62-3
- Eltrombopag
Catalog No.:BCC4968
CAS No.:496775-61-2
- H-D-Phe(4-Me)-OH
Catalog No.:BCC3271
CAS No.:49759-61-7
- Stiripentol
Catalog No.:BCC3977
CAS No.:49763-96-4
- 11beta-Hydroxylupeol
Catalog No.:BCN7571
CAS No.:49776-92-3
- AEE788 (NVP-AEE788)
Catalog No.:BCC2520
CAS No.:497839-62-0
- Vanillyl alcohol
Catalog No.:BCN3832
CAS No.:498-00-0
- Acetovanillone
Catalog No.:BCN2916
CAS No.:498-02-2
- 1,6-Anhydro-β-D-glucose
Catalog No.:BCC8427
CAS No.:498-07-7
- Scopine
Catalog No.:BCN1940
CAS No.:498-45-3
- Ethyl Nipecotate
Catalog No.:BCC3272
CAS No.:5006-62-2
- Tobramycin Sulfate
Catalog No.:BCC5633
CAS No.:49842-07-1
- EX 527 (SEN0014196)
Catalog No.:BCC2223
CAS No.:49843-98-3
- Erythroskyrin
Catalog No.:BCN1836
CAS No.:4987-27-3
Effects of dobutamine hydrochloride on cardiovascular function in horses anesthetized with isoflurane with or without acepromazine maleate premedication.[Pubmed:27901396]
Am J Vet Res. 2016 Dec;77(12):1318-1324.
OBJECTIVE To determine the effects of acepromazine maleate premedication on cardiovascular function before and after infusion of Dobutamine hydrochloride for 30 minutes in isoflurane-anesthetized horses. ANIMALS 6 healthy adult horses. PROCEDURES Each horse was anesthetized once following premedication with acepromazine (0.02 mg/kg, IV) administered 30 minutes prior to anesthetic induction (ACP+ treatment) and once without premedication (ACP- treatment). Anesthesia was induced with IV administration of xylazine hydrochloride (0.8 mg/kg), ketamine hydrochloride (2.2 mg/kg), and diazepam (0.08 mg/kg). Horses were positioned in right lateral recumbency, and anesthesia was maintained via inhalation of isoflurane delivered in oxygen. End-tidal isoflurane concentration was adjusted to achieve a target mean arterial blood pressure of 60 mm Hg (interquartile range [25th to 75th percentile], 57 to 63 mm Hg) for at least 15 minutes. Cardiac index, oxygen delivery index, and femoral arterial blood flow indices were determined 60 minutes after anesthetic induction (baseline). Dobutamine was then infused to achieve a target mean arterial blood pressure of 80 mm Hg (interquartile range, 76 to 80 mm Hg). Data collection was repeated 30 minutes after the start of dobutamine infusion for comparison with baseline values. RESULTS Complete data sets were available from 5 of the 6 horses. Dobutamine administration resulted in significant increases in oxygen delivery and femoral arterial blood flow indices but no significant change in cardiac index for each treatment. However, at baseline or 30 minutes after the start of dobutamine infusion, findings for the ACP+ and ACP- treatments did not differ. CONCLUSIONS AND CLINICAL RELEVANCE In isoflurane-anesthetized horses, dobutamine administration increased oxygen delivery and femoral arterial blood flow indices, but these changes were unaffected by premedication with acepromazine.
Hydrogen profiles of dobutamine hydrochloride and fentanyl citrate solutions according to intravenous administration systems, temperature, and luminosity conditions.[Pubmed:25191819]
J Infus Nurs. 2014 Sep-Oct;37(5):362-8.
Factors such as temperature, light exposure, drug concentration, ionic strength, time of infusion, and duration of drug association can influence the effectiveness of pharmacological solutions, which can compromise the solutions' quality, resulting in unstable solutions and drug incompatibility. The aim of this study was to determine the pH of solutions of Dobutamine hydrochloride, fentanyl citrate, and their combination in 5% dextrose in water (D5W) under various light exposures and temperature conditions over time. The analysis was performed by measuring the pH of the substances in both pharmacological (commercial) preparations and in D5W under dark fluorescent light in the presence or absence of sunlight exposures, intravenous apparatus packaging (clear and amber burettes), and temperature (22 degrees C and 37 degrees C). Samples were collected immediately after preparation and after 0.5, 1, 2, 3, 4, and 24 hours of exposure to the various conditions; data were analyzed using mean standard deviations. Of the 260 pH values obtained, 50 (19.2%) were from commercial preparations and 210 (80.8%) from solutions exposed to various experimental conditions. Significant pH differences were found among the vials of the commercial preparation drugs. The largest pH value difference (0.88) was observed for fentanyl citrate, in which a pH increase of 0.88 (4.23 +/- 0.62) was observed. The combination of drugs in D5W resulted in more acidic values than those of fentanyl citrate and of D5W and fentanyl citrate in D5W, but they were closer to what was observed for the solution of Dobutamine hydrochloride in D5W. This solution was more acidic than fentanyl citrate diluted in D5W. The lower acidity of fentanyl citrate had a minor influence on the final pH of the combined drug solution in D5W. Under most conditions, the drug solutions kept at 22 degrees C had pH values that were more acidic and less variable. Temperature was a major factor controlling the chemical behavior of the solutions analyzed. Analysis of chemical behavior in response to light exposure indicated that the solutions were more stable over time when kept in the dark.
Determination of dobutamine hydrochloride by enzymatic catalytic spectrofluorimetry.[Pubmed:23616473]
Luminescence. 2014 Feb;29(1):92-5.
A highly sensitive and simple spectrofluorimetric method for the determination of Dobutamine hydrochloride based on its inhibitory effect on the hemoglobin-catalyzed reaction of H2 O2 and l-tyrosine was developed. The relationship between the concentration of Dobutamine hydrochloride and the fluorescence quenching (DeltaF) of the system is linear under the optimal experimental conditions. The calibration graph is linear in the range 2.00 x 10(-7) to 3.00 x 10(-6) g/mL with a limit of detection of 4.83 x 10(-9) g/mL. This method can be used for the determination of Dobutamine hydrochloride in its pharmaceutical formulations and in urine with satisfactory results.
Effect of intravenous infusion of dobutamine hydrochloride on the development of early postoperative cognitive dysfunction in elderly patients via inhibiting the release of tumor necrosis factor-alpha.[Pubmed:25131356]
Eur J Pharmacol. 2014 Oct 15;741:150-5.
To investigate the effects of Dobutamine hydrochloride on early postoperative cognitive dysfunction (POCD) and plasma tumor necrosis factor (TNF)-alpha concentration in patients undergoing hip arthroplasty, 124 patients undergoing unilateral total hip arthroplasty, aged 70-92 years old, were randomly assigned to four groups (n=31) as follows: a control group of patients receiving only saline (intravenous infusion, i.v.); and groups receiving 2, 4, or 6mugkg(-1)min(-1) (i.v.) of Dobutamine hydrochloride. Cognitive functions were assessed on the day before surgery (T1), and the 1st day (T2), 3rd day (T3), and 7th day (T4) postsurgery using the Mini Mental State Examination (MMSE). The plasma TNF-alpha protein level was determined 10min before anesthesia (Ta), and 10min (Tb), 30min (Tc), and 60min (Td) after anesthesia by an enzyme-linked immunosorbent assay. Cognitive disorder was observed within the first 3 days after hip arthroplastic surgery, and it had recovered 7 days after the operation in the control group of patients. Administration of 2 or 4mugkg(-1)min(-1) Dobutamine hydrochloride was able to reverse the early POCD. Simultaneously, an increase of plasma TNF-alpha levels 30min after anesthesia was observed (41.34+/-9.61 vs. 27.75+/-5.45), which was significantly suppressed by the administration of low-dose Dobutamine hydrochloride (29.23+/-7.32 vs. 41.34+/-9.61) but not by high-dose Dobutamine hydrochloride (45.9+/-12.11 vs. 41.34+/-9.61). Together, our data indicated that the plasma concentration of TNFalpha was engaged in the effect of Dobutamine hydrochloride on POCD.
Characterization of the beta adrenoceptor subtype(s) mediating the positive inotropic effects of epinine, dopamine, dobutamine, denopamine and xamoterol in isolated human right atrium.[Pubmed:1354251]
J Pharmacol Exp Ther. 1992 Aug;262(2):532-8.
In patients with chronic heart failure cardiac beta-1 adrenoceptors are reduced, whereas beta-2 adrenoceptor changes vary depending on the etiology of the disease. Beta Adrenoceptor agonists can be used for short-term inotropic support in chronic heart failure; their clinical efficacy might depend on which beta adrenoceptor subtype(s) mediates their positive inotropic effect. Thus, the beta adrenoceptor subtype(s) involved in the positive inotropic effects of clinically used beta adrenoceptor agonists was characterized on isolated electrically driven human right atria by the use of the selective beta-1 adrenoceptor antagonist CGP 20712 A (300 nmol/l) and/or the selective beta-2 adrenoceptor antagonist ICI 118,551 (30 nmol/l). Epinine evoked positive inotropic effects through stimulation of beta-1 and beta-2 adrenoceptors to about the same degree, whereas dobutamine acted mainly at beta-1 adrenoceptors but had a significant beta-2 adrenoceptor component. Both agonists were full agonists causing the same maximum increase in contractile force (Emax) as did isoprenaline or Ca++ (Emax = 1.0). In contrast, denopamine was a partial selective beta-1 adrenoceptor agonist (Emax = 0.75-0.85). Dopamine was in the presence of uptake1-blockade (by 5 mumol/l phenoxybenzamine) a partial agonist (Emax = 0.60-0.70) acting selectively at beta-1 adrenoceptors; in the absence of uptake1-blockade, however, dopamine was a full agonist, indicating that part of its positive inotropic effect is indirect via the release of endogenous noradrenaline. Xamoterol did not exert positive inotropic effects, but concentration-dependently slightly decreased basal force of contraction.
Dobutamine: positive inotropy by nonselective adrenoceptor agonism in isolated guinea pig and human myocardium.[Pubmed:3600817]
Naunyn Schmiedebergs Arch Pharmacol. 1987 Apr;335(4):385-90.
Positive inotropic responses to dobutamine have been examined using isolated myocardium from guinea pigs und humans. The potency (EC50) of dobutamine was 1.5 X 10(-6) mol/l on guinea pig papillary muscles, 1.8 X 10(-6) mol/l on guinea pig left atria and 2.5 X 10(-6) mol/l on human papillary muscle strips. In guinea pig cardiac muscles, Schild plots for the beta 1-selective antagonist, 1-practolol, using dobutamine as agonist, had slopes of less than unity. This suggested the involvement of other receptors in the inotropic response to dobutamine. The beta 2-selective antagonist, ICI 118,551, but not the alpha 1-selective antagonist, prazosin, attenuated the dobutamine response in guinea pig papillary muscles. Both ICI 118,551 and prazosin shifted the dobutamine concentration-response curve in guinea pig left atria. The positive inotropic response to dobutamine in human papillary muscles was antagonised by l-practolol and ICI 118,551 but not by prazosin. The maximal inotropic response to dobutamine was 90% that of calcium measured in the same guinea pig papillary muscles but only 37% that of calcium in human papillary muscle strips. This reduced maximal effect of dobutamine in human myocardium is probably a disease-induced change but species variations cannot be excluded.


