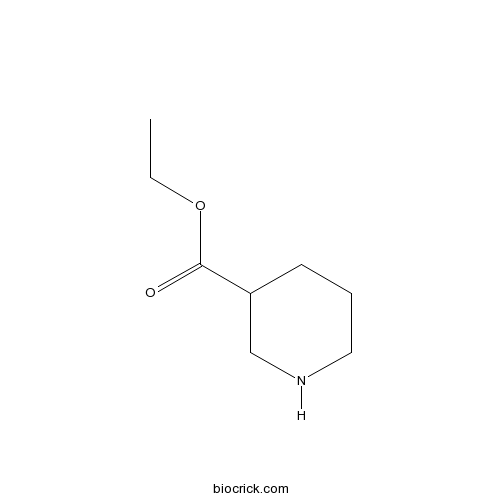
Ethyl NipecotateCAS# 5006-62-2 |
- Hydroxyfasudil
Catalog No.:BCC1635
CAS No.:105628-72-6
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- Hydroxyfasudil hydrochloride
Catalog No.:BCC1636
CAS No.:155558-32-0
- H-1152 dihydrochloride
Catalog No.:BCC1616
CAS No.:871543-07-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5006-62-2 | SDF | Download SDF |
| PubChem ID | 98969 | Appearance | Powder |
| Formula | C8H15NO2 | M.Wt | 157.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ethyl piperidine-3-carboxylate;Ethyl 3-piperidinecarboxylate;Nipecotic acid ethyl ester;71962-74-8 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | ethyl piperidine-3-carboxylate | ||
| SMILES | CCOC(=O)C1CCCNC1 | ||
| Standard InChIKey | XIWBSOUNZWSFKU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H15NO2/c1-2-11-8(10)7-4-3-5-9-6-7/h7,9H,2-6H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ethyl Nipecotate Dilution Calculator

Ethyl Nipecotate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.3613 mL | 31.8066 mL | 63.6132 mL | 127.2265 mL | 159.0331 mL |
| 5 mM | 1.2723 mL | 6.3613 mL | 12.7226 mL | 25.4453 mL | 31.8066 mL |
| 10 mM | 0.6361 mL | 3.1807 mL | 6.3613 mL | 12.7226 mL | 15.9033 mL |
| 50 mM | 0.1272 mL | 0.6361 mL | 1.2723 mL | 2.5445 mL | 3.1807 mL |
| 100 mM | 0.0636 mL | 0.3181 mL | 0.6361 mL | 1.2723 mL | 1.5903 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Scopine
Catalog No.:BCN1940
CAS No.:498-45-3
- 1,6-Anhydro-β-D-glucose
Catalog No.:BCC8427
CAS No.:498-07-7
- Acetovanillone
Catalog No.:BCN2916
CAS No.:498-02-2
- Vanillyl alcohol
Catalog No.:BCN3832
CAS No.:498-00-0
- AEE788 (NVP-AEE788)
Catalog No.:BCC2520
CAS No.:497839-62-0
- 11beta-Hydroxylupeol
Catalog No.:BCN7571
CAS No.:49776-92-3
- Stiripentol
Catalog No.:BCC3977
CAS No.:49763-96-4
- H-D-Phe(4-Me)-OH
Catalog No.:BCC3271
CAS No.:49759-61-7
- Dobutamine hydrochloride
Catalog No.:BCC5391
CAS No.:49745-95-1
- Tupichinol A
Catalog No.:BCN7697
CAS No.:497142-88-8
- DC_AC50
Catalog No.:BCC6488
CAS No.:497061-48-0
- Z-Thr-NH2
Catalog No.:BCC2738
CAS No.:49705-98-8
- Tobramycin Sulfate
Catalog No.:BCC5633
CAS No.:49842-07-1
- EX 527 (SEN0014196)
Catalog No.:BCC2223
CAS No.:49843-98-3
- Erythroskyrin
Catalog No.:BCN1836
CAS No.:4987-27-3
- Corydamine
Catalog No.:BCN3366
CAS No.:49870-84-0
- IsoMaltose
Catalog No.:BCN8321
CAS No.:499-40-1
- beta-Thujaplicin
Catalog No.:BCN3895
CAS No.:499-44-5
- 5-Isopropyl-2-methylphenol
Catalog No.:BCN2633
CAS No.:499-75-2
- 2,4-Pyridinedicarboxylic Acid
Catalog No.:BCC6483
CAS No.:499-80-9
- Tioxolone
Catalog No.:BCC2316
CAS No.:4991-65-5
- Guanidine HCl
Catalog No.:BCC4785
CAS No.:50-01-1
- Dexamethasone (DHAP)
Catalog No.:BCC1184
CAS No.:50-02-2
- Cortisone acetate
Catalog No.:BCC4771
CAS No.:50-04-4
Expression of spinal cord GABA transporter 1 in morphine-tolerant male Wistar rats.[Pubmed:26463038]
Eur J Pharmacol. 2015 Nov 15;767:77-81.
Chronic morphine exposure produces morphine tolerance. One of the mechanisms of morphine tolerance involves gamma-aminobutric acid (GABA), whose level is regulated by GABA transporter 1 (GAT-1). The aim of this study was to investigate the expression of GAT-1 in the spinal cord during morphine treatment. Morphine was administrated to rats via drinking water for 21 days. On day 21, a single dose of morphine (10mg/kg) was injected, followed by the administration of 5% formalin after 30 min. Expression of GAT-1 in the lumbar spinal cord during morphine treatment was analyzed by Western blotting and immunohistochemistry assay. In another set of experiments, a morphine-tolerant group was treated with a GAT-1 inhibitor, Ethyl Nipecotate (60 mg/kg), 5 min prior to the formalin test. To assess a possible analgesic effect of the GAT-1 inhibitor, a non-tolerant group was injected only with Ethyl Nipecotate 5 min prior to the formalin test. Our results indicated that a chronic consumption of morphine led to morphine tolerance. Morphine tolerance was also concomitant with GAT-1 up-regulation in the lumbar spinal cord. The GAT-1 inhibitor Ethyl Nipecotate improved the antinociceptive effect of morphine in the morphine-tolerant group. Ethyl Nipecotate also had an antinociceptive effect on the non-tolerant group. Thus, our data suggest that GAT-1 overexpression in the spinal cord plays an important role in morphine tolerance.
Increase in drug-induced seizure susceptibility of transgenic mice overexpressing GABA transporter-1.[Pubmed:14531940]
Acta Pharmacol Sin. 2003 Oct;24(10):991-5.
AIM: The changes of seizure susceptibility of transgenic mice overexpressing GABA transporter-1 (GAT-1) were studied to clarify the possible role of GABAergic transmission in epileptogenesis. METHODS: Seizures were induced by intraperitoneal administration of pentylenetetrazol (PTZ), picrotoxin (PIC), or kainic acid (KA) respectively. The anticonvulsant effect of Ethyl Nipecotate was tested by its intraperitoneal injection 15 min before the administration of the epileptogenic agents. RESULTS: The percentages of occurrence of clonic seizures induced by PTZ 45 mg/kg, PIC 2.5 mg/kg, or KA 20 mg/kg in GAT-1 transgenic mice were 88.9 %, 100 %, and 83.3 % respectively, whereas those in control C57BL/6J mice were 42.9 %, 57.1 %, and 33.3 %. The percentages of occurrence of tonic seizures induced by PTZ 45 mg/kg, PIC 2.5 mg/kg, or KA 20 mg/kg in transgenic mice were 88.9 %, 100 %, and 83.3 % respectively, and whereas those in control mice were 28.6 %, 42.9 %, and 16.7 %. The latencies of both clonic and tonic seizures onset in transgenic mice were markedly shortened compared with those in control animals. The results indicated that GAT-1 transgenic mice showed increased susceptibility to seizures induced by the anti-GABAergic convulsive drugs (PTZ, PIC), as well as glutamic receptor agonist (KA). Ethyl Nipecotate, inhibitor of GAT-1, inhibited PTZ-induced seizures in both GAT-1 transgenic and C57BL/6J mice. The incidence of seizures was decreased after the application of Ethyl Nipecotate, and the latencies to the onset of clonic or tonic seizures were also prolonged. CONCLUSION: The increase in seizure susceptibility of transgenic mice over-expressing GAT-1 is an evidence for involvement of GABAergic transmission in epileptogenesis, and this transgenic mouse might be a useful animal model for study on the role of GABAergic transmission in epileptogenesis.
Hyperalgesic effects of gamma-aminobutyric acid transporter I in mice.[Pubmed:12898541]
J Neurosci Res. 2003 Aug 15;73(4):565-72.
The present study focused on the involvement of gamma-aminobutyric acid transporter I (GAT1) in pain. We found that GABA uptake was increased in mouse spinal cord at 20 min and 120 min after formalin injection and in mouse brain at 120 min, but not 20 min, after formalin injection. In addition, the antinociceptive effects of GAT1-selective inhibitors were examined using assays of thermal (tail-flick) and chemical (formalin and acetic acid) nociception in C57BL/6J mice. The GAT1-selective inhibitors, Ethyl Nipecotate and NO-711, exhibited significant antinociceptive effects in these nociceptive assays. To study further the effects of GAT1 on pain, we used two kinds of GAT1-overexpressing transgenic mice (under the control of a CMV promoter or a NSE promoter) to examine the nociceptive responses in these mice. In the thermal, formalin, and acetic acid assays, both kinds of transgenic mice displayed significant hyperalgesia after nociceptive stimuli. In addition, the micro opioid receptor antagonist naloxone had no influence on nociceptive responses in wild-type and transgenic mice. The results indicate that GAT1 is involved in the regulation of pain processes, and point to the possibility of developing analgesic drugs that target GAT1 other than opioid receptors.
Separation of the S(+) and R(-)-enantiomers of tiagabine.HCl and its two chiral precursors by chiral chromatography: application to chiral inversion studies.[Pubmed:9800663]
J Pharm Biomed Anal. 1998 Sep;17(8):1439-47.
Chiral HPLC methods were developed and validated for tiagabine.HCl and its two chiral precursors to determine the chiral purity of the three compounds to ensure the quality of the final product which is used as a new antiepileptic drug. Tiagabine.HCl was derivatized with 1-napthalenemethylamine and was chromatographed on a Pirkle type phenyl glycine column with a mobile phase of 69:31, 0.1 M ammonium acetateacetonitrile (v/v). The two chiral precursors were chromatographed on a Chiralcel-OG column with a mobile phase of hexane, isopropanol etc. Each of the three HPLC methods have a selectivity factor (alpha) of 1-2 or higher. The validation of the methods was done by conducting standard addition and recovery studies of the S(+)-enantiomers in the samples. The %RSD of all three methods were < 5 with a limit of quantification of 0.05% (peak area) or lower. By using these methods, a study was conducted to investigate the effect of pH, temperature, and trace levels of transition metals such as Fe3+, Co2+, and Ni2+ on the conversion of R(-)-enantiomer to the S(+)-enantiomer of tiagabine.HCl and its two chiral precursors. The results of this study demonstrated that the two chiral precursors of tiagabine.HCl under reflux conditions are more sensitive to chiral inversion than tiagabine.HCl. Under reflux conditions, in the presence of trace metal ions and different pH, approximately 10, 11, and 1% of the R(-)-enantiomer was converted to the S(+)-enantiomer for Ethyl Nipecotate, ethylester of tiagabine, and tiagabine.HCl, respectively. However, at room temperature, tiagabine.HCl appears to be less chirally stable than its two chiral precursors. Approximately 0.4% R(-)-enantiomer of tiagabine.HCl was converted to the S(+)-enantiomer at room temerature and acidic conditions. Under similar conditions, the S(+)-enantiomer of Ethyl Nipecotate and ethylester of tiagabine.HCl was < 0.05%. The initial S(+)-enantiomer content for all three compounds was < 0.1%.
Synthesis of stereoisomers of antithrombotic nipecotamides.[Pubmed:7742174]
Chirality. 1995;7(2):90-5.
The stereoisomers of alpha,alpha'-bis[3-(N,N-diethylcarbamoyl)-piperidino]-p-xylene (1) were synthesized. Rac Ethyl Nipecotate was resolved by diastereomeric (-)-D- and (+)-L-tartrate salt formation. The enantiomeric esters were hydrolyzed to the corresponding nipecotic acids, which were then converted into t-BOC derivatives. Treatment of the latter with diethylamine/isobutyl chloroformate and removal of the t-BOC protecting group afforded (R)- and (S)-N,N-diethylnipecotamides. Condensation of the latter with alpha,alpha'-dibromo-p-xylene gave (R,R)- and (S,S)-1. The meso-diastereomer was obtained by stereospecific synthesis in addition to our earlier procedure involving fractional crystallization of the diastereomeric mixture obtained by synthesis. The latter was resolved earlier into 1A, 1B, and 1C using chiral high-performance liquid chromatography (HPLC). Based on the stereospecific synthesis now achieved, 1A and 1B are assigned the configurations, (R,R) and (S,S) respectively, and 1C is assigned the meso configuration. The (R,S) structure of the latter is also confirmed by X-ray crystallography.
A comparison of prodrug esters of nipecotic acid.[Pubmed:3393269]
Neuropharmacology. 1988 May;27(5):475-83.
The relative ability of the enantiomers of the ethyl and m-nitrophenyl esters of nipecotic acid to block convulsions induced by bicuculline and pentylenetetrazol, as well as to block the uptake of GABA into whole brain mini-slices, was studied in mice. Neither (+)Ethyl Nipecotate hydrogen tartrate [(+)E.Tartrate], which is hydrolyzed to (-)nipecotic acid, nor (-)Ethyl Nipecotate hydrogen tartrate [(-)E.Tartrate], which is hydrolyzed to (+)nipecotic acid, provided protection against challenge with bicuculline. Both (+)E.Tartrate and (-)Ethyl Nipecotate hydrochloride [(-)E.HCl], which are hydrolyzed to (-)nipecotic acid, blocked seizures induced by pentylenetetrazol. However, neither (-)E.Tartrate nor (+)Ethyl Nipecotate hydrochloride [(+)E.HCl], which are hydrolyzed to (+)nipecotic acid, provided significant protection against challenge with pentylenetetrazol. These results agree with the relative ability of these compounds to inhibit the uptake of GABA, where (-)nipecotic acid was more potent than (+)nipecotic acid and (+)E.Tartrate was more potent than (-)E.Tartrate. The enantiomers of m-nitrophenyl-3-piperidinecarboxylate hydrochloride, (+)MNPC.HCl and (-)MNPC.HCl, were almost equi-effective in preventing seizures induced by bicuculline. This lack of significant difference in anticonvulsant activity is in contrast with the ability to inhibit the uptake of GABA, where (-)MNPC.HCl was significantly more potent than (+)MNPC.HCl. Changing the route of administration from subcutaneous to intraperitoneal injection reduced the onset of time of the peak effect and the anticonvulsant potency of (+/-)MNPC.HCl. Cholinergic effects were observed with the administration of (+)E.Tartrate and (-)E.HCl, but not with (-)E.Tartrate, (+)E.HCl, (+)MNPC.HCl or (-)MNPC.HCl.(ABSTRACT TRUNCATED AT 250 WORDS)


