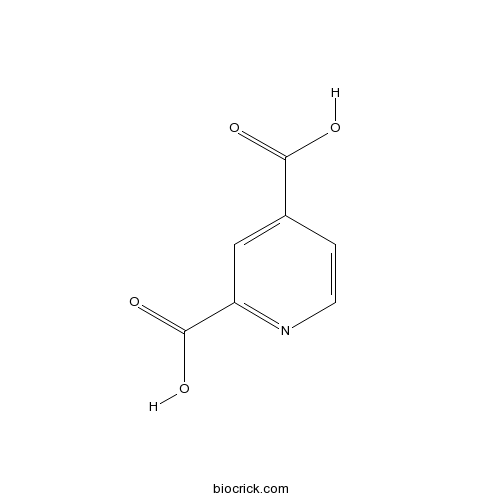
2,4-Pyridinedicarboxylic AcidHistone LSD inhibitor CAS# 499-80-9 |
- (24R)-MC 976
Catalog No.:BCC1289
CAS No.:112828-09-8
- (24S)-MC 976
Catalog No.:BCC1291
CAS No.:112849-14-6
- 1alpha, 25-Dihydroxy VD2-D6
Catalog No.:BCC1299
CAS No.:216244-04-1
- 1alpha, 24, 25-Trihydroxy VD2
Catalog No.:BCC1298
CAS No.:457048-34-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 499-80-9 | SDF | Download SDF |
| PubChem ID | 10365 | Appearance | Powder |
| Formula | C7H5NO4 | M.Wt | 167.12 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >9.15mg/mL in DMSO | ||
| Chemical Name | pyridine-2,4-dicarboxylic acid | ||
| SMILES | C1=CN=C(C=C1C(=O)O)C(=O)O | ||
| Standard InChIKey | MJIVRKPEXXHNJT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H5NO4/c9-6(10)4-1-2-8-5(3-4)7(11)12/h1-3H,(H,9,10)(H,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2,4-Pyridinedicarboxylic Acid Dilution Calculator

2,4-Pyridinedicarboxylic Acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.9837 mL | 29.9186 mL | 59.8372 mL | 119.6745 mL | 149.5931 mL |
| 5 mM | 1.1967 mL | 5.9837 mL | 11.9674 mL | 23.9349 mL | 29.9186 mL |
| 10 mM | 0.5984 mL | 2.9919 mL | 5.9837 mL | 11.9674 mL | 14.9593 mL |
| 50 mM | 0.1197 mL | 0.5984 mL | 1.1967 mL | 2.3935 mL | 2.9919 mL |
| 100 mM | 0.0598 mL | 0.2992 mL | 0.5984 mL | 1.1967 mL | 1.4959 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
2,4-Pyridinedicarboxylic Acid (2,4-PDCA) is an inhibitor of histone lysine-specific demethylases that targets on JMJD2A (KDM4A), KDM4C, KDM4E (IC50, 1.4 μM), KDM5B (IC50, 3 μM), KDM6A and other 2-oxogynases [1][2].
Histone lysine-specific demethylases JMJD2A (KDM4A) and KDM4C are both members of the Jumonji domain 2 (JMJD2) family and function as trimethylation-specific demethylases, converting specific trimethylated histone residues to the dimethylated form. KDM5B is an H3K4me3⁄ me2-specific lysine demethylase [1][2].
2,4-Pyridinedicarboxylic Acid (2,4-PDCA) is an inhibitor of JMJD2A (KDM4A), KDM4C and KDM5B. In the FDH-coupled assay, 2,4-PDCA inhibited ccKDM5B with IC50 value of 3 ± 1 μM. In MALDI-TOF analysis of H3(1-21)K4me3, 2,4-PDCA reduced the level of H3(1-15) induced by ccKDM5B. In U2-OS cells transfected with KDM5B, 2,4-PDCA inhibited the decrease of H3K4me3 [1]. In HG-treated VSMCs, 2,4-PDCA (1.0 mM) inhibited JMJD2A and HG-induced proliferation in a concentration-dependent way, and inhibited HG-induced migration. 2,4-PDCA also reduced the mRNA and protein levels of MCP-1 and IL-6 [2].
In diabetic rats, 2,4-PDCA (7.5 mg/kg/d) reduced neointimal area and I/M ratio in the injured arteries 28 days after injury. 2,4-PDCA also inhibited the percentage of PCNA-positive cells in the neointima [2].
References:
[1]. Kristensen LH, Nielsen AL, Helgstrand C, et al. Studies of H3K4me3 demethylation by KDM5B/Jarid1B/PLU1 reveals strong substrate recognition in vitro and identifies 2,4-pyridine-dicarboxylic acid as an in vitro and in cell inhibitor. FEBS J, 2012, 279(11): 1905-1914.
[2]. Qi H, Jing Z, Xiaolin W, et al. Histone Demethylase JMJD2A Inhibition Attenuates Neointimal Hyperplasia in the Carotid Arteries of Balloon-Injured Diabetic Rats via Transcriptional Silencing: Inflammatory Gene Expression in Vascular Smooth Muscle Cells. Cell Physiol Biochem, 2015, 37(2): 719-734.
- 5-Isopropyl-2-methylphenol
Catalog No.:BCN2633
CAS No.:499-75-2
- beta-Thujaplicin
Catalog No.:BCN3895
CAS No.:499-44-5
- IsoMaltose
Catalog No.:BCN8321
CAS No.:499-40-1
- Corydamine
Catalog No.:BCN3366
CAS No.:49870-84-0
- Erythroskyrin
Catalog No.:BCN1836
CAS No.:4987-27-3
- EX 527 (SEN0014196)
Catalog No.:BCC2223
CAS No.:49843-98-3
- Tobramycin Sulfate
Catalog No.:BCC5633
CAS No.:49842-07-1
- Ethyl Nipecotate
Catalog No.:BCC3272
CAS No.:5006-62-2
- Scopine
Catalog No.:BCN1940
CAS No.:498-45-3
- 1,6-Anhydro-β-D-glucose
Catalog No.:BCC8427
CAS No.:498-07-7
- Acetovanillone
Catalog No.:BCN2916
CAS No.:498-02-2
- Vanillyl alcohol
Catalog No.:BCN3832
CAS No.:498-00-0
- Tioxolone
Catalog No.:BCC2316
CAS No.:4991-65-5
- Guanidine HCl
Catalog No.:BCC4785
CAS No.:50-01-1
- Dexamethasone (DHAP)
Catalog No.:BCC1184
CAS No.:50-02-2
- Cortisone acetate
Catalog No.:BCC4771
CAS No.:50-04-4
- Mitomycin C
Catalog No.:BCC2388
CAS No.:50-07-7
- Ergocalciferol
Catalog No.:BCN2208
CAS No.:50-14-6
- Cyclophosphamide
Catalog No.:BCC1185
CAS No.:50-18-0
- Corticosterone
Catalog No.:BCN2203
CAS No.:50-22-6
- Hydrocortisone
Catalog No.:BCN2192
CAS No.:50-23-7
- Prednisolone
Catalog No.:BCC4830
CAS No.:50-24-8
- Estriol
Catalog No.:BCN2235
CAS No.:50-27-1
- beta-Estradiol
Catalog No.:BCN2194
CAS No.:50-28-2
A red zwitterionic co-crystal of acetaminophen and 2,4-pyridinedicarboxylic acid.[Pubmed:20574998]
J Pharm Sci. 2010 Sep;99(9):3676-83.
We report on a co-crystal of acetaminophen (APAP) and 2,4-Pyridinedicarboxylic Acid (PDA). The co-crystal was discovered by screening using the solution-mediated phase transformation (SMPT) technique. Despite the bulk solids of each component being white in color, the new co-crystal phase exhibited a red color. The new phase was analyzed using single-crystal X-ray diffraction and identified as (APAP).(PDA).(1). Structural analysis revealed PDA to exist in a hitherto unreported zwitterionic form in the co-crystal. A structural analysis of pure PDA revealed the presence of the zwitterion form in (PDA).(H(2)O) (2), as well. The components of 1 self-assemble as a three-dimensional (3D) hydrogen-bonded network with a pronounced 2D structure. The origin of the red color was investigated using density functional theory calculations, which demonstrate a decreasing pi-pi(*) separation involving the components of the solid.
Investigations on the synthesis and properties of N-aryl (heteroaryl) piperazinylalkyl derivatives of imide of 6-methyl-2-(1-piperidino)-3,4-pyridinedicarboxylic acid.[Pubmed:7945715]
Farmaco. 1994 Jul-Aug;49(7-8):493-8.
The synthesis of N-aryl(heteroaryl)piperazinylalkyl derivatives of imide of 2-(1-piperidino)-3,4-pyridinedicarboxylic acid is reported. 3 examined compounds proved to be active pharmacologically in some tests.


