ErgocalciferolDNA Polymerase inhibitor CAS# 50-14-6 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 50-14-6 | SDF | Download SDF |
| PubChem ID | 6536972 | Appearance | Powder |
| Formula | C28H44O | M.Wt | 396.65 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | Ergocalciferol; Calciferol; Ercalciol | ||
| Solubility | Soluble in DMSO > 10 mM | ||
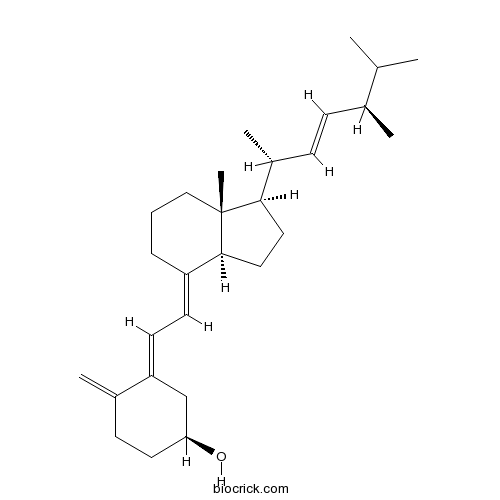
| Chemical Name | (1S,3E)-3-[(2E)-2-[(1R,3aS,7aR)-1-[(E,2R,5R)-5,6-dimethylhept-3-en-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidenecyclohexan-1-ol | ||
| SMILES | CC(C)C(C)C=CC(C)C1CCC2C1(CCCC2=CC=C3CC(CCC3=C)O)C | ||
| Standard InChIKey | MECHNRXZTMCUDQ-VLOQVYPSSA-N | ||
| Standard InChI | InChI=1S/C28H44O/c1-19(2)20(3)9-10-22(5)26-15-16-27-23(8-7-17-28(26,27)6)12-13-24-18-25(29)14-11-21(24)4/h9-10,12-13,19-20,22,25-27,29H,4,7-8,11,14-18H2,1-3,5-6H3/b10-9+,23-12+,24-13+/t20-,22+,25-,26+,27-,28+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ergocalciferol (Vitamin D2) is a selective inhibitor of mammalian DNA polymerase A (pol A) with IC50 of 123 mM. Ergocalciferol can delay the development of secondary hyperparathyroidism in children with CKD2-3. Ergocalciferol can cause HL-60 apoptosis via a modulation of mitochondria involving ROS production, GSH depletion, caspase activation, and Fas induction, on the basis of anticancer activity of ergocalciferol, it may be feasible to develop chemopreventive agents from edible mushrooms or hop. Ergocalciferol can prevent glucocorticoid-induced bone loss, but even increase lumbar spine and femoral neck bone mineral densities (BMD) in postmenopausal women commencing glucocorticoid therapy, it also is safe and effective in reducing the risk of a fracture in elderly patients with AD. |
| Targets | IL Receptor | Caspase | ROS | DNA polymerase A | GSH | Fas | PARP |
| In vivo | Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism: a randomized trial.[Pubmed: 22266572 ]Clin J Am Soc Nephrol. 2012 Feb;7(2):216-23.Vitamin D deficiency is an important contributor to the development of hyperparathyroidism and is independently associated with cardiovascular and bone disease. The hypothesis was that nutritional vitamin D (Ergocalciferol) supplementation in children with CKD stages 2-4 delays the onset of secondary hyperparathyroidism.
Ergocalciferol decreases erythropoietin resistance in children with chronic kidney disease stage 5.[Pubmed: 23420502]Pediatr Nephrol. 2013 Aug;28(8):1261-6.Vitamin D(Ergocalciferol) insufficiency is related to erythropoietin resistance in chronic kidney disease (CKD). This study was conducted to evaluate the effect of Ergocalciferol on the dose of erythrocyte-stimulating agent (ESA) administered to children with CKD stage 5 and vitamin D insufficiency.
Ergocalciferol treatment and aspects of mineral homeostasis in patients with chronic kidney disease stage 4-5.[Pubmed: 23281842]Scand J Clin Lab Invest. 2013 Mar;73(2):107-16.Focus on non-classical effects and possible less side effects of treatment with nutritional vitamin D, raises the expectation of possible benefits from treating chronic kidney disease (CKD) patients with Ergocalciferol (vitamin D2). Treatment with 1,25(OH)2 vitamin D (calcitriol) induces elevated fibroblast growth factor 23 (FGF23), while epidemiological studies have found positive effects of nutritional and 25(OH)vitamin D on mortality in CKD. Disturbed mineral homeostasis in CKD is correlated to adverse outcome and cardiovascular mortality. The objective was to examine the possible effects of treatment with high doses of Ergocalciferol on parameters of mineral homeostasis in predialysis CKD patients.
Cyclical etidronate plus ergocalciferol prevents glucocorticoid-induced bone loss in postmenopausal women.[Pubmed: 7733124]Am J Med. 1995 May;98(5):459-63.The two groups did not differ with respect to age, years since the menopause, mean daily To assess the benefit of cyclical etidronate plus Ergocalciferol for the prevention of glucocorticoid-induced bone loss in a 2-year, prospective, open study based in an osteoporosis clinic.
|
| Cell Research | Induction of apoptosis by vitamin D2, ergocalciferol, via reactive oxygen species generation, glutathione depletion, and caspase activation in human leukemia Cells.[Pubmed: 18386902 ]J Agric Food Chem. 2008 May 14;56(9):2996-3005.This study demonstrated that Ergocalciferol was able to inhibit leukemia cell growth in a concentration-dependent manner.
|
| Animal Research | Ergocalciferol promotes in vivo differentiation of keratinocytes and reduces photodamage caused by ultraviolet irradiation in hairless mice.[Pubmed: 15379869 ]Photodermatol Photoimmunol Photomed. 2004 Oct;20(5):215-23.Ergocalciferol (VD(2)) is usually administered orally and it is metabolized to produce its biologically active metabolites in the liver and kidney. Active vitamin D is a well-known potent regulator of cell growth and differentiation.
Active vitamin D such as 1,25-dihydroxyvitamin D(3) (1alpha,25(OH)(2)D(3)) prevents photodamage, including wrinkles and morphologic alterations. However, its clinical and cosmetic use is limited because of its potent, associated effect on calcium metabolism. We examined the efficacy of vitamin D analogues with few adverse effects for preventing skin photodamage.
|

Ergocalciferol Dilution Calculator

Ergocalciferol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5211 mL | 12.6056 mL | 25.2111 mL | 50.4223 mL | 63.0279 mL |
| 5 mM | 0.5042 mL | 2.5211 mL | 5.0422 mL | 10.0845 mL | 12.6056 mL |
| 10 mM | 0.2521 mL | 1.2606 mL | 2.5211 mL | 5.0422 mL | 6.3028 mL |
| 50 mM | 0.0504 mL | 0.2521 mL | 0.5042 mL | 1.0084 mL | 1.2606 mL |
| 100 mM | 0.0252 mL | 0.1261 mL | 0.2521 mL | 0.5042 mL | 0.6303 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Vitamin D2 has a strong inhibitory effect against bladder tumor promotion by sodium saccharin and induces cell differentiation in leukemia cells. Vitamin D2 is an inhibitor of DNA Polymerase.
- Mitomycin C
Catalog No.:BCC2388
CAS No.:50-07-7
- Cortisone acetate
Catalog No.:BCC4771
CAS No.:50-04-4
- Dexamethasone (DHAP)
Catalog No.:BCC1184
CAS No.:50-02-2
- Guanidine HCl
Catalog No.:BCC4785
CAS No.:50-01-1
- Tioxolone
Catalog No.:BCC2316
CAS No.:4991-65-5
- 2,4-Pyridinedicarboxylic Acid
Catalog No.:BCC6483
CAS No.:499-80-9
- 5-Isopropyl-2-methylphenol
Catalog No.:BCN2633
CAS No.:499-75-2
- beta-Thujaplicin
Catalog No.:BCN3895
CAS No.:499-44-5
- IsoMaltose
Catalog No.:BCN8321
CAS No.:499-40-1
- Corydamine
Catalog No.:BCN3366
CAS No.:49870-84-0
- Erythroskyrin
Catalog No.:BCN1836
CAS No.:4987-27-3
- EX 527 (SEN0014196)
Catalog No.:BCC2223
CAS No.:49843-98-3
- Cyclophosphamide
Catalog No.:BCC1185
CAS No.:50-18-0
- Corticosterone
Catalog No.:BCN2203
CAS No.:50-22-6
- Hydrocortisone
Catalog No.:BCN2192
CAS No.:50-23-7
- Prednisolone
Catalog No.:BCC4830
CAS No.:50-24-8
- Estriol
Catalog No.:BCN2235
CAS No.:50-27-1
- beta-Estradiol
Catalog No.:BCN2194
CAS No.:50-28-2
- Phenylbutazone
Catalog No.:BCC4822
CAS No.:50-33-9
- Thalidomide
Catalog No.:BCC2248
CAS No.:50-35-1
- Cocaine
Catalog No.:BCN1901
CAS No.:50-36-2
- Clomiphene citrate
Catalog No.:BCC4480
CAS No.:50-41-9
- Mercaptopurine (6-MP)
Catalog No.:BCC1186
CAS No.:50-44-2
- Estradiol Benzoate
Catalog No.:BCC4779
CAS No.:50-50-0
Ergocalciferol treatment and aspects of mineral homeostasis in patients with chronic kidney disease stage 4-5.[Pubmed:23281842]
Scand J Clin Lab Invest. 2013 Mar;73(2):107-16.
BACKGROUND: Focus on non-classical effects and possible less side effects of treatment with nutritional vitamin D, raises the expectation of possible benefits from treating chronic kidney disease (CKD) patients with Ergocalciferol (vitamin D2). Treatment with 1,25(OH)2 vitamin D (calcitriol) induces elevated fibroblast growth factor 23 (FGF23), while epidemiological studies have found positive effects of nutritional and 25(OH)vitamin D on mortality in CKD. Disturbed mineral homeostasis in CKD is correlated to adverse outcome and cardiovascular mortality. The objective was to examine the possible effects of treatment with high doses of Ergocalciferol on parameters of mineral homeostasis in predialysis CKD patients. METHODS: A total of 43 adult patients with CKD stage 4-5, not receiving vitamin D supplementation, were studied, and allocated by simple randomization to either an intervention (n = 26) or a control group (n = 17). The intervention group received Ergocalciferol, 50.000 IU/week for 6 weeks. Plasma FGF23, creatinine, parathyroid hormone (PTH), phosphate and ionized calcium were obtained at baseline and after the 6 weeks. RESULTS: The intervention group had a significant increase in 25(OH)D2 concentration from < 10 to 90 +/- 4 nmol/L, while 1,25(OH)2D (62 +/- 6 at baseline and 67 +/- 6 pmol/L at 6 weeks) remained stable. No changes were seen in the circulating vitamin D concentrations in the control group. After the 6 weeks of treatment no significant changes were seen in concentration of creatinine, phosphate, ionized calcium, PTH and FGF23 remained stable. CONCLUSION: No harmful effects of short-term treatment with high-dose Ergocalciferol were observed on markers of mineral homeostasis and FGF23 in CKD patients stage 4-5.
Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism: a randomized trial.[Pubmed:22266572]
Clin J Am Soc Nephrol. 2012 Feb;7(2):216-23.
BACKGROUND AND OBJECTIVES: Vitamin D deficiency is an important contributor to the development of hyperparathyroidism and is independently associated with cardiovascular and bone disease. The hypothesis was that nutritional vitamin D (Ergocalciferol) supplementation in children with CKD stages 2-4 delays the onset of secondary hyperparathyroidism. DESIGN, SETTING, PARTICIPANTS, & MEASUREMENTS: A randomized, double-blinded, placebo-controlled study in children with CKD2-4 who had 25-hydroxyvitamin D [25(OH)D] deficiency was conducted. Ergocalciferol (or a matched placebo) was given daily as per Kidney Disease Outcomes Quality Initiative guidelines. The primary endpoint was the time to development of hyperparathyroidism. RESULTS: Seventy-two children were screened. Forty-seven children were 25(OH)D-deficient and randomly assigned to receive Ergocalciferol or placebo. Twenty children in each arm completed the study; median follow-up was 12 months. Groups were well matched for age, race, estimated GFR, and season when recruited. Nine of 20 children on placebo and 3 of 20 children on Ergocalciferol developed hyperparathyroidism (odds ratio, 4.64; 95% confidence interval, 1.02-21.00). The time to development of hyperparathyroidism was significantly longer with Ergocalciferol treatment compared with placebo (hazard ratio, 0.30; 95% confidence interval, 0.09-0.93, P=0.05). With Ergocalciferol treatment, normal 25(OH)D levels were achieved in all 8 children with CKD2, 8 of 11 children with CKD3, but not in the single patient with CKD4. There were no Ergocalciferol-related adverse events. 25(OH)D levels >100 nmol/L were required to achieve normal levels of 1,25-dihydroxyvitamin D. CONCLUSIONS: Ergocalciferol is an effective treatment that delays the development of secondary hyperparathyroidism in children with CKD2-3.
Ergocalciferol promotes in vivo differentiation of keratinocytes and reduces photodamage caused by ultraviolet irradiation in hairless mice.[Pubmed:15379869]
Photodermatol Photoimmunol Photomed. 2004 Oct;20(5):215-23.
BACKGROUND: Ergocalciferol (VD(2)) is usually administered orally and it is metabolized to produce its biologically active metabolites in the liver and kidney. Active vitamin D is a well-known potent regulator of cell growth and differentiation. PURPOSE: Active vitamin D such as 1,25-dihydroxyvitamin D(3) (1alpha,25(OH)(2)D(3)) prevents photodamage, including wrinkles and morphologic alterations. However, its clinical and cosmetic use is limited because of its potent, associated effect on calcium metabolism. We examined the efficacy of vitamin D analogues with few adverse effects for preventing skin photodamage. METHOD: Topical application of VD(2) to hairless mouse dorsal skin, and exposure to solar-simulating ultraviolet (UV) radiation at a dose of 10.8 J/cm(2) (UVA) were performed for 15 weeks, five times a week on weekdays. At the end of the final irradiation, histological and analytical studies were performed. RESULTS: Topical application of VD(2) significantly prevented wrinkle formation and abnormal accumulation of extracellular matrix components. In addition, VD(2) suppressed excessive secretion of IL-6 induced by UV irradiation in cultured human normal keratinocytes, in a dose-dependent manner. CONCLUSION: VD(2) promoted keratinocytes differentiation in the epidermis and showed diverse physiological effects, the same as the active form of VD(3). The results suggested that the suppression of skin photodamage involved the promotion of keratinocytes differentiation and suppression of IL-6 secretion induced by exposure to UV. Topical application of VD(2) may become an effective means to suppress solar UV-induced human skin damage.
Induction of apoptosis by vitamin D2, ergocalciferol, via reactive oxygen species generation, glutathione depletion, and caspase activation in human leukemia Cells.[Pubmed:18386902]
J Agric Food Chem. 2008 May 14;56(9):2996-3005.
This study demonstrated that Ergocalciferol was able to inhibit leukemia cell growth in a concentration-dependent manner. Exploration of the acting mechanisms involved this event revealed that Ergocalciferol induced DNA fragmentation and increased sub-G1 DNA contents in HL-60 cells, both of which are hallmarks of apoptosis. Analysis of the integrity of mitochondria demonstrated that Ergocalciferol caused loss of mitochondrial membrane potential with release cytochrome c to cytosol, generation of reactive oxygen species (ROS), and depletion of glutathione (GSH), suggesting that Ergocalciferol may induce apoptosis in HL-60 cells through a ROS-dependent pathway. Further results show that caspases-2, -3, -6, and -9 were all activated by Ergocalciferol, together with cleavage of the downstream caspase-3 targets, DNA fragmentation factor (DFF-45), and poly(ADP-ribose) polymerase. In addition, Ergocalciferol led to the increase in pro-apoptotic factor Bax accompanied with the decrease in anti-apoptotic member Mcl-1, and the reduced Mcl-1 to Bax ratio may be a critical event concerning mitochondrial decay by Ergocalciferol. Furthermore, Ergocalciferol also led to induction of Fas death receptor closely linked to caspase-2 activation, suggesting the involvement of a Fas-mediated pathway in Ergocalciferol-induced apoptosis. Totally, these findings suggest that Ergocalciferol causes HL-60 apoptosis via a modulation of mitochondria involving ROS production, GSH depletion, caspase activation, and Fas induction. On the basis of anticancer activity of Ergocalciferol, it may be feasible to develop chemopreventive agents from edible mushrooms or hop.
Ergocalciferol decreases erythropoietin resistance in children with chronic kidney disease stage 5.[Pubmed:23420502]
Pediatr Nephrol. 2013 Aug;28(8):1261-6.
BACKGROUND: Vitamin D insufficiency is related to erythropoietin resistance in chronic kidney disease (CKD). This study was conducted to evaluate the effect of Ergocalciferol on the dose of erythrocyte-stimulating agent (ESA) administered to children with CKD stage 5 and vitamin D insufficiency. METHODS: Twenty patients aged <18 years with CKD stages 5 or 5D and vitamin D insufficiency were divided into two groups. During the 12-week study, ten patients received oral Ergocalciferol (treatment) whereas the other ten patients did not (control). The ESA dosage was recorded monthly. RESULTS: There were no significant differences in demographic data, ESA dosages, and laboratory data, including corrected calcium, phosphorus, parathyroid hormone, hemoglobin, ferritin, 25-hydroxyvitamin D (25D), and transferrin saturation levels, between the two groups at baseline. At the completion of the study, serum 25D levels in the treatment group were significantly increased from baseline (p = 0.02) and were significantly higher than the serum 25D levels in the controls (p < 0.005). The ESA dosage in the treatment group was significantly decreased when compared to baseline (p = 0.04). CONCLUSIONS: Vitamin D deficiency should be routinely detected and treated. Our results show that the administration of Ergocalciferol in conjunction with 1,25-dihydroxyvitamin D3 reduced the dose of ESA required to treat children with CKD stages 5 and 5D and may decrease erythropoietin resistance.
Cyclical etidronate plus ergocalciferol prevents glucocorticoid-induced bone loss in postmenopausal women.[Pubmed:7733124]
Am J Med. 1995 May;98(5):459-63.
OBJECTIVE: To assess the benefit of cyclical etidronate plus Ergocalciferol for the prevention of glucocorticoid-induced bone loss in a 2-year, prospective, open study based in an osteoporosis clinic. PATIENTS AND METHODS: Group 1 consisted of 15 postmenopausal women (mean age 62.6 +/- 3.3 years) who commenced glucocorticoid therapy and were treated with cyclical etidronate (400 mg/d for the first month; thereafter, 400 mg/d for 2 weeks of every 3-month period), elemental calcium (1 g/d), and Ergocalciferol (0.5 mg/wk). Group 2 consisted of 11 postmenopausal women (mean age 60.2 +/- 4.7 years) with glucocorticoid-induced osteoporosis, who were attending the clinic at the same time and were treated with calcium supplements only (1 g/d). MEASUREMENTS: Lumbar spine and femoral neck bone mineral densities (BMD) were measured at baseline and after 12 and 24 months of glucocorticoid therapy using a dual energy x-ray absorptiometer. RESULTS: The two groups did not differ with respect to age, years since the menopause, mean daily glucocorticoid dose, and baseline BMD values. During the first year of therapy, mean lumbar spine BMD increased from an initial value of 0.88 g/cm2 to 0.94 g/cm2, an increase of 7% per year (95% confidence interval [CI] 3.7% to 10.2%; P < 0.001 compared with controls). Significant increases in BMD of 2.5% per year were also observed in the femoral neck (95% CI -1% to 6%; P < 0.01 compared with controls). After the second year of cyclical etidronate therapy, femoral neck BMD continued to increase (P < 0.05 compared with value at 12 months), while lumbar spine BMD remained stable. CONCLUSION: Chronic glucocorticoid therapy may result in bone loss at most skeletal sites. Therapy with cyclical etidronate plus Ergocalciferol not only prevented glucocorticoid-induced bone loss, but even increased lumbar spine and femoral neck BMD in postmenopausal women commencing glucocorticoid therapy.


