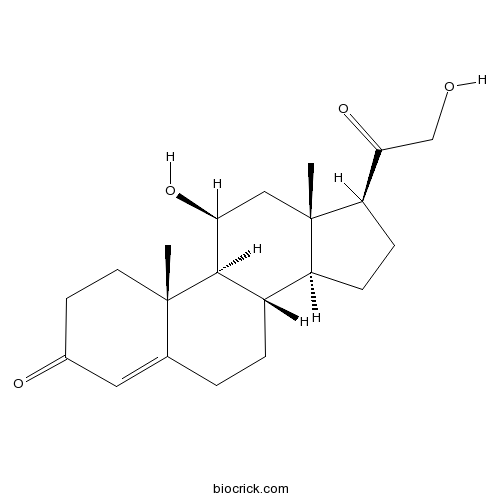
CorticosteroneEndogenous glucocorticoid CAS# 50-22-6 |
- CZC24832
Catalog No.:BCC1507
CAS No.:1159824-67-5
- PI3Kγ inhibitor 1
Catalog No.:BCC4180
CAS No.:1172118-03-4
- CAL-130
Catalog No.:BCC1440
CAS No.:1431697-74-3
- A-443654
Catalog No.:BCC1321
CAS No.:552325-16-3
- CAL-101 (Idelalisib, GS-1101)
Catalog No.:BCC1270
CAS No.:870281-82-6
- BKM120
Catalog No.:BCC1279
CAS No.:944396-07-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 50-22-6 | SDF | Download SDF |
| PubChem ID | 5753 | Appearance | Powder |
| Formula | C21H30O4 | M.Wt | 346.46 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Synonyms | 17-Deoxycortisol; 11β,21-Dihydroxyprogesterone; Kendall's compound B | ||
| Solubility | DMSO : 50 mg/mL (144.32 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | (8S,9S,10R,11S,13S,14S,17S)-11-hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one | ||
| SMILES | CC12CCC(=O)C=C1CCC3C2C(CC4(C3CCC4C(=O)CO)C)O | ||
| Standard InChIKey | OMFXVFTZEKFJBZ-HJTSIMOOSA-N | ||
| Standard InChI | InChI=1S/C21H30O4/c1-20-8-7-13(23)9-12(20)3-4-14-15-5-6-16(18(25)11-22)21(15,2)10-17(24)19(14)20/h9,14-17,19,22,24H,3-8,10-11H2,1-2H3/t14-,15-,16+,17-,19+,20-,21-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Corticosterone, the major stress hormone, is an adrenocortical steroid that has modest but significant activities as a mineralocorticoid and a glucocorticoid, it is cytotoxic toward neurons, and the cytotoxic effect could be protected by Yokukansan. A delayed effect of elevated Corticosterone on breeding success rather than an immediate suppression of prolactin concentrations causing premature failure. |
| Targets | IL Receptor | TNF-α | AChR |
| In vivo | Protracted treatment with corticosterone reduces breeding success in a long-lived bird.[Pubmed: 25449182]Gen Comp Endocrinol. 2015 Jan 1;210:38-45.Determining the physiological mechanisms underpinning life-history decisions is essential for understanding the constraints under which life-history strategies can evolve. In long-lived species, where the residual reproductive value of breeders is high, adult survival is a key contributor to lifetime reproductive success. We therefore expect that when adult survival is compromised during reproduction, mechanisms will evolve to redirect resources away from reproduction, with implications for reproductive hormones, adult body mass, nest attendance behaviour and breeding success.
Corticosterone primes the neuroinflammatory response to DFP in mice: potential animal model of Gulf War Illness.[Pubmed: 25753028]J Neurochem. 2015 Jun;133(5):708-21.Gulf War Illness (GWI) is a multi-symptom disorder with features characteristic of persistent sickness behavior. Among conditions encountered in the Gulf War (GW) theater were physiological stressors (e.g., heat/cold/physical activity/sleep deprivation), prophylactic treatment with the reversible AChE inhibitor, pyridostigmine bromide (PB), the insect repellent, N,N-diethyl-meta-toluamide (DEET), and potentially the nerve agent, sarin. Prior exposure to the anti-inflammatory glucocorticoid, Corticosterone (CORT), at levels associated with high physiological stress, can paradoxically prime the CNS to produce a robust proinflammatory response to neurotoxicants and systemic inflammation; such neuroinflammatory effects can be associated with sickness behavior.
|
| Cell Research | Neuroprotective effect of yokukansan against cytotoxicity induced by corticosterone on mouse hippocampal neurons.[Pubmed: 25022209]Phytomedicine. 2014 Sep 25;21(11):1458-65.Yokukansan, a traditional Japanese herbal medicine, has been used for the management of neurodegenerative disorders and for the treatment of neurosis, insomnia, and behavioral and psychological symptoms of dementia. Recently, several studies have shown that yokukansan has a neuroprotective effect.
|
| Animal Research | Stress hormone corticosterone enhances susceptibility to cortical spreading depression in familial hemiplegic migraine type 1 mutant mice.[Pubmed: 25447936]Exp Neurol. 2015 Jan;263:214-20.Stress is a putative migraine trigger, but the pathogenic mechanisms involved are unknown. Stress and stress hormones increase neuronal excitability by enhancing glutamatergic neurotransmission, but inhibitory effects have also been reported.
|

Corticosterone Dilution Calculator

Corticosterone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8863 mL | 14.4317 mL | 28.8634 mL | 57.7267 mL | 72.1584 mL |
| 5 mM | 0.5773 mL | 2.8863 mL | 5.7727 mL | 11.5453 mL | 14.4317 mL |
| 10 mM | 0.2886 mL | 1.4432 mL | 2.8863 mL | 5.7727 mL | 7.2158 mL |
| 50 mM | 0.0577 mL | 0.2886 mL | 0.5773 mL | 1.1545 mL | 1.4432 mL |
| 100 mM | 0.0289 mL | 0.1443 mL | 0.2886 mL | 0.5773 mL | 0.7216 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Corticosterone is a sensitive inhibitor of monoamine transport. Target: OCT(organic cation transporter) Corticosterone is a 21-carbon steroid hormone of the corticosteroid type produced in the cortex of the adrenal glands in rodents and other non-human animals. Corticosterone inhibits organic cation transport via some OCTs in peripheral tissues. Corticosterone also inhibits efflux of [ 3H]-MPP from hypothalamic minces.[1]
References:
[1]. Gasser PJ, et al. Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organiccation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J Neurosci. 2006 Aug 23;26(34):8758-8766.
- Cyclophosphamide
Catalog No.:BCC1185
CAS No.:50-18-0
- Ergocalciferol
Catalog No.:BCN2208
CAS No.:50-14-6
- Mitomycin C
Catalog No.:BCC2388
CAS No.:50-07-7
- Cortisone acetate
Catalog No.:BCC4771
CAS No.:50-04-4
- Dexamethasone (DHAP)
Catalog No.:BCC1184
CAS No.:50-02-2
- Guanidine HCl
Catalog No.:BCC4785
CAS No.:50-01-1
- Tioxolone
Catalog No.:BCC2316
CAS No.:4991-65-5
- 2,4-Pyridinedicarboxylic Acid
Catalog No.:BCC6483
CAS No.:499-80-9
- 5-Isopropyl-2-methylphenol
Catalog No.:BCN2633
CAS No.:499-75-2
- beta-Thujaplicin
Catalog No.:BCN3895
CAS No.:499-44-5
- IsoMaltose
Catalog No.:BCN8321
CAS No.:499-40-1
- Corydamine
Catalog No.:BCN3366
CAS No.:49870-84-0
- Hydrocortisone
Catalog No.:BCN2192
CAS No.:50-23-7
- Prednisolone
Catalog No.:BCC4830
CAS No.:50-24-8
- Estriol
Catalog No.:BCN2235
CAS No.:50-27-1
- beta-Estradiol
Catalog No.:BCN2194
CAS No.:50-28-2
- Phenylbutazone
Catalog No.:BCC4822
CAS No.:50-33-9
- Thalidomide
Catalog No.:BCC2248
CAS No.:50-35-1
- Cocaine
Catalog No.:BCN1901
CAS No.:50-36-2
- Clomiphene citrate
Catalog No.:BCC4480
CAS No.:50-41-9
- Mercaptopurine (6-MP)
Catalog No.:BCC1186
CAS No.:50-44-2
- Estradiol Benzoate
Catalog No.:BCC4779
CAS No.:50-50-0
- Reserpine
Catalog No.:BCN4960
CAS No.:50-55-5
- Oxytocin
Catalog No.:BCC5435
CAS No.:50-56-6
Protracted treatment with corticosterone reduces breeding success in a long-lived bird.[Pubmed:25449182]
Gen Comp Endocrinol. 2015 Jan 1;210:38-45.
Determining the physiological mechanisms underpinning life-history decisions is essential for understanding the constraints under which life-history strategies can evolve. In long-lived species, where the residual reproductive value of breeders is high, adult survival is a key contributor to lifetime reproductive success. We therefore expect that when adult survival is compromised during reproduction, mechanisms will evolve to redirect resources away from reproduction, with implications for reproductive hormones, adult body mass, nest attendance behaviour and breeding success. We investigated whether manipulating Corticosterone, to simulate exposure to an environmental stressor, affected the secretion of prolactin and breeding success in the black-legged kittiwake Rissa tridactyla. We used implanted Alzet(R) osmotic pumps to administer Corticosterone to incubating kittiwakes at a constant rate over a period of approximately 8days. Manipulated birds were compared with sham implanted birds and control birds, which had no implants. There was no significant difference in the body mass of captured individuals at the time of implantation and implant removal. Corticosterone-implanted males showed lower nest attendance during the chick rearing period compared to sham-implanted males; the opposite pattern was found in females. Corticosterone treated birds showed a marginally significant reduction in breeding success compared to sham-implanted individuals, with all failures occurring at least 1week after implant removal. However, prolactin concentrations at implant removal were not significantly different from initial values. We were unable to measure the profile of change in Corticosterone during the experiment. However, our results suggest a delayed effect of elevated Corticosterone on breeding success rather than an immediate suppression of prolactin concentrations causing premature failure.
Corticosterone primes the neuroinflammatory response to DFP in mice: potential animal model of Gulf War Illness.[Pubmed:25753028]
J Neurochem. 2015 Jun;133(5):708-21.
Gulf War Illness (GWI) is a multi-symptom disorder with features characteristic of persistent sickness behavior. Among conditions encountered in the Gulf War (GW) theater were physiological stressors (e.g., heat/cold/physical activity/sleep deprivation), prophylactic treatment with the reversible AChE inhibitor, pyridostigmine bromide (PB), the insect repellent, N,N-diethyl-meta-toluamide (DEET), and potentially the nerve agent, sarin. Prior exposure to the anti-inflammatory glucocorticoid, Corticosterone (CORT), at levels associated with high physiological stress, can paradoxically prime the CNS to produce a robust proinflammatory response to neurotoxicants and systemic inflammation; such neuroinflammatory effects can be associated with sickness behavior. Here, we examined whether CORT primed the CNS to mount neuroinflammatory responses to GW exposures as a potential model of GWI. Male C57BL/6 mice were treated with chronic (14 days) PB/ DEET, subchronic (7-14 days) CORT, and acute exposure (day 15) to diisopropyl fluorophosphate (DFP), a sarin surrogate and irreversible AChE inhibitor. DFP alone caused marked brain-wide neuroinflammation assessed by qPCR of tumor necrosis factor-alpha, IL6, chemokine (C-C motif) ligand 2, IL-1beta, leukemia inhibitory factor, and oncostatin M. Pre-treatment with high physiological levels of CORT greatly augmented (up to 300-fold) the neuroinflammatory responses to DFP. Anti-inflammatory pre-treatment with minocycline suppressed many proinflammatory responses to CORT+DFP. Our findings are suggestive of a possible critical, yet unrecognized interaction between the stressor/environment of the GW theater and agent exposure(s) unique to this war. Such exposures may in fact prime the CNS to amplify future neuroinflammatory responses to pathogens, injury, or toxicity. Such occurrences could potentially result in the prolonged episodes of sickness behavior observed in GWI. Gulf War (GW) veterans were exposed to stressors, prophylactic medicines and, potentially, nerve agents in theater. Subsequent development of GW Illness, a persistent multi-symptom disorder with features characteristic of sickness behavior, may be caused by priming of the CNS resulting in exaggerated neuroinflammatory responses to pathogens/insults. Nerve agent, diisopropyl fluorophosphate (DFP), produced a neuroinflammatory response that was exacerbated by pre-treatment with levels of Corticosterone simulating heightened stressor conditions. While prophylactic treatments reduced DFP-induced neuroinflammation, this effect was negated when those treatments were combined with Corticosterone.
Stress hormone corticosterone enhances susceptibility to cortical spreading depression in familial hemiplegic migraine type 1 mutant mice.[Pubmed:25447936]
Exp Neurol. 2015 Jan;263:214-20.
Stress is a putative migraine trigger, but the pathogenic mechanisms involved are unknown. Stress and stress hormones increase neuronal excitability by enhancing glutamatergic neurotransmission, but inhibitory effects have also been reported. We hypothesise that an acute rise in stress hormones, such as corticosteroids which are released after stress, increase neuronal excitability and thereby may increase susceptibility to cortical spreading depression (CSD), the mechanism underlying the migraine aura. Here we investigated effects of acute restraint stress and of the stress hormone Corticosterone on CSD susceptibility as surrogate migraine marker, in a transgenic mouse model of familial hemiplegic migraine type 1 (FHM1), which displays increased glutamatergic cortical neurotransmission and increased propensity for CSD. We found that 20-min and 3-h restraint stress did not influence CSD susceptibility in mutant or wild-type mice, despite elevated levels of plasma Corticosterone. By contrast, subcutaneous administration of 20mg/kg Corticosterone increased CSD frequency exclusively in mutant mice, while Corticosterone plasma levels were similarly elevated in mutants and wild types. The effect of Corticosterone on CSD frequency was normalised by pre-administration of the glucocorticoid receptor (GR) antagonist mifepristone. These findings suggest that corticosteroid-induced GR activation can enhance susceptibility to CSD in genetically susceptible individuals, and may predispose to attacks of migraine. Although Corticosterone levels rise also during acute stress, the latter likely triggers a spatiotemporally more complex biological response with multiple positive and negative modulators which may not be adequately modeled by exogenous administration of Corticosterone alone.
Neuroprotective effect of yokukansan against cytotoxicity induced by corticosterone on mouse hippocampal neurons.[Pubmed:25022209]
Phytomedicine. 2014 Sep 25;21(11):1458-65.
Yokukansan, a traditional Japanese herbal medicine, has been used for the management of neurodegenerative disorders and for the treatment of neurosis, insomnia, and behavioral and psychological symptoms of dementia. Recently, several studies have shown that yokukansan has a neuroprotective effect. The aim of this study was to examine the neuroprotective effect of yokukansan on hippocampal neurons from embryonic mouse brain against the effects of Corticosterone, which is considered to be a stress hormone and to be cytotoxic toward neurons. The cell survival rates were measured by the WST-8 assay and LDH assay. Twenty-four hours after treatment with Corticosterone, cell numbers were significantly decreased compared with the control or treatment with vehicle in a dose-dependent manner. When cells were treated with 30 muM Corticosterone, the decrease in the number of cells was significantly recovered by treatment with yokukansan (100-1,000 mug/ml) in a dose-dependent manner. However, yokukansan did not suppress the decrease in cell numbers that was induced by treatment with 100 muM Corticosterone. In the LDH assay, treatment with yokukansan at a high concentration (500-1,000 mug/ml) suppressed the LDH concentration induced by treatment with both 30 muM and 100 muM Corticosterone compared to treatment with Corticosterone alone, respectively. These results suggest that yokukansan protects against the cytotoxic effect of a low concentration of Corticosterone on hippocampal neurons.


