ThalidomideImmunomodulatory agent,sedative drug,angiogenesis inhibitor CAS# 50-35-1 |
- Lenalidomide hydrochloride
Catalog No.:BCC1697
CAS No.:1243329-97-6
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- Necrostatin 2 racemate
Catalog No.:BCC2077
CAS No.:852391-15-2
- Necrostatin 2
Catalog No.:BCC1793
CAS No.:852391-19-6
- Necrostatin 2 S enantiomer
Catalog No.:BCC2078
CAS No.:852391-20-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 50-35-1 | SDF | Download SDF |
| PubChem ID | 5426 | Appearance | Powder |
| Formula | C13H10N2O4 | M.Wt | 258.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (193.63 mM; Need ultrasonic) | ||
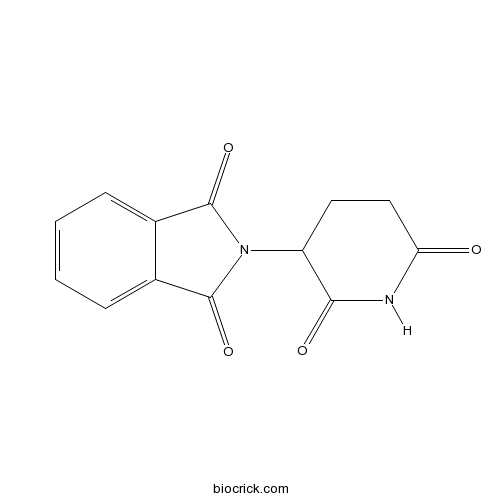
| Chemical Name | 2-(2,6-dioxopiperidin-3-yl)isoindole-1,3-dione | ||
| SMILES | C1CC(=O)NC(=O)C1N2C(=O)C3=CC=CC=C3C2=O | ||
| Standard InChIKey | UEJJHQNACJXSKW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H10N2O4/c16-10-6-5-9(11(17)14-10)15-12(18)7-3-1-2-4-8(7)13(15)19/h1-4,9H,5-6H2,(H,14,16,17) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Teratogen, sedative-hypnotic with inherent anti-inflammatory properties. A selective inhibitor of tumor necrosis factor α (TNF-α) synthesis. |

Thalidomide Dilution Calculator

Thalidomide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8725 mL | 19.3626 mL | 38.7252 mL | 77.4503 mL | 96.8129 mL |
| 5 mM | 0.7745 mL | 3.8725 mL | 7.745 mL | 15.4901 mL | 19.3626 mL |
| 10 mM | 0.3873 mL | 1.9363 mL | 3.8725 mL | 7.745 mL | 9.6813 mL |
| 50 mM | 0.0775 mL | 0.3873 mL | 0.7745 mL | 1.549 mL | 1.9363 mL |
| 100 mM | 0.0387 mL | 0.1936 mL | 0.3873 mL | 0.7745 mL | 0.9681 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Thalidomide was introduced as a sedative drug,immunomodulatory agent and also is investigated for treating symptoms of many cancers.Thalidomide inhibits an E3 ubiquitin ligase, which is a CRBN-DDB1-Cul4A complex.
- Phenylbutazone
Catalog No.:BCC4822
CAS No.:50-33-9
- beta-Estradiol
Catalog No.:BCN2194
CAS No.:50-28-2
- Estriol
Catalog No.:BCN2235
CAS No.:50-27-1
- Prednisolone
Catalog No.:BCC4830
CAS No.:50-24-8
- Hydrocortisone
Catalog No.:BCN2192
CAS No.:50-23-7
- Corticosterone
Catalog No.:BCN2203
CAS No.:50-22-6
- Cyclophosphamide
Catalog No.:BCC1185
CAS No.:50-18-0
- Ergocalciferol
Catalog No.:BCN2208
CAS No.:50-14-6
- Mitomycin C
Catalog No.:BCC2388
CAS No.:50-07-7
- Cortisone acetate
Catalog No.:BCC4771
CAS No.:50-04-4
- Dexamethasone (DHAP)
Catalog No.:BCC1184
CAS No.:50-02-2
- Guanidine HCl
Catalog No.:BCC4785
CAS No.:50-01-1
- Cocaine
Catalog No.:BCN1901
CAS No.:50-36-2
- Clomiphene citrate
Catalog No.:BCC4480
CAS No.:50-41-9
- Mercaptopurine (6-MP)
Catalog No.:BCC1186
CAS No.:50-44-2
- Estradiol Benzoate
Catalog No.:BCC4779
CAS No.:50-50-0
- Reserpine
Catalog No.:BCN4960
CAS No.:50-55-5
- Oxytocin
Catalog No.:BCC5435
CAS No.:50-56-6
- Chloroquine diphosphate
Catalog No.:BCC3915
CAS No.:50-63-5
- Niclosamide
Catalog No.:BCC5081
CAS No.:50-65-7
- 5-Hydroxytryptamine
Catalog No.:BCC9204
CAS No.:50-67-9
- 1,3:2,4-Di-p-methylbenyliedene sorbitol
Catalog No.:BCC4847
CAS No.:54686-97-4
- Actinomycin D
Catalog No.:BCC2385
CAS No.:50-76-0
- Aspirin (Acetylsalicylic acid)
Catalog No.:BCC2097
CAS No.:50-78-2
Results from Two Consecutive Studies of Consolidation Therapy after Autologous Transplant for Multiple Myeloma: Thalidomide, Dexamethasone, and Clarithromycin or Lenalidomide, Dexamethasone, and Clarithromycin.[Pubmed:28355602]
Acta Haematol. 2017;137(3):123-131.
BACKGROUND: In multiple myeloma (MM), relapse is a problem after autologous hematopoietic stem cell transplantation (ASCT). In the nontransplant setting, Thalidomide/dexamethasone/clarithromycin (BLT-D) and lenalidomide/dexamethasone/clarithromycin (BiRd) achieve responses with acceptable toxicity. Both regimens are reasonable objects of study in the post-ASCT setting. PATIENTS AND METHODS: We report on BLT-D and BiRd given post-ASCT. Studies were conducted consecutively. After recovery from ASCT, therapy was started. All 3 drugs were given for 1 year, and then immunomodulatory drugs alone were given as long as tolerated or until disease progression. RESULTS: For BLT-D, the most common toxicity was peripheral neuropathy (PN). For BiRd, infection, PN, and neutropenia were the most common adverse events. BiRd was associated with a higher frequency of secondary cancers. The median follow-up for BLT-D was 10.2 years (range 8.6-10.7) and for BiRd it was 7.5 years (range 6.4-8.4). After BLT-D, 18 patients (67%) were alive and 10 (37%) were alive without disease progression, and after BiRd, 18 patients (58%) were alive and 10 (32%) were alive without disease progression. CONCLUSIONS: BLT-D and BiRd can be given post-ASCT with different toxicity profiles and comparable disease-free and overall survival rates. A randomized study comparing these regimens to single-agent lenalidomide is needed to determine which approach is superior. Key Message: Relapse of MM is a major problem after ASCT. Strategies are needed post-ASCT to improve outcomes. In the nontransplant setting, Thalidomide or lenalidomide/dexamethasone/clarithromycin treat MM with acceptable toxicity. We, thus, studied both regimens post- ASCT. They can be given with different toxicity profiles and result in good disease control.
Endoscopic and Histologic Healing in Children With Inflammatory Bowel Diseases Treated With Thalidomide.[Pubmed:28286192]
Clin Gastroenterol Hepatol. 2017 Sep;15(9):1382-1389.e1.
BACKGROUND & AIMS: Mucosal healing, determined by endoscopic evaluation, is one of the most important prognostic markers for patients with inflammatory bowel diseases. Findings from histologic evaluation, however, could complement findings from endoscopy in assessing mucosal responses to treatment. We analyzed long-term results of children treated with Thalidomide to determine the association between clinical response and histology and endoscopy findings. METHODS: We collected data from 2 multicenter trials of 70 children with refractory Crohn's disease (CD) or ulcerative colitis (UC) (2-18 years old; ileocolonic or colonic disease) given Thalidomide or placebo (NCT00720538). Clinical remission and clinical response at 8 weeks were defined as a pediatric CD activity index scores 10 points or lower and a decrease of at least 50% from baseline, respectively, for patients with CD; and as a pediatric UC activity index score below 10 and a decrease of at least 20 points from baseline, respectively, for patients with UC. Patients with a clinical response to 8 weeks' treatment with Thalidomide underwent endoscopic examination with biopsy collection at study weeks 12 and 52. Severity of inflammation in patients with UC was assessed by Mayo score and in patients with CD by 4-grade system. Biopsies were assessed for signs of active inflammation, erosion or ulceration, and crypt abscesses and assigned a histologic score. RESULTS: Clinical remission was observed in 42 patients (60.0%) and clinical response in 45 patients (64.2%) at Week 8. At Week 52, a total of 38 patients (54.3%) were still in clinical remission or still had a clinical response; 29 patients (41.4%) had mucosal healing, defined as complete healing of erosions or ulcerations, and 20 patients (27.7%) had histologic healing, defined as complete absence of markers of inflammation. Of patients with clinical remission or clinical response, 75.3% also had mucosal healing and 52.6% also had histologic healing. The probability of achieving mucosal healing decreased significantly with increasing values of erythrocyte sedimentation rate (adjusted odds ratio, 0.96; 95% CI, 0.93-0.98; P = .006). CONCLUSIONS: In a long-term analysis of data from 2 clinical trials of pediatric patients with CD or UC, 52 weeks' treatment with Thalidomide led to clinical remission in 54.3% of patients with ileocolonic or colonic disease; of these patients, 75.3% had mucosal healing and 52.6% also had histologic healing. Further studies are needed to determine how Thalidomide therapy affects long-term progression of inflammatory bowel diseases. (ClinicalTrials.gov number NCT00720538).
Thalidomide influences growth and vasculogenic mimicry channel formation in melanoma.[Pubmed:18983651]
J Exp Clin Cancer Res. 2008 Nov 4;27:60.
AIMS: To observe the effects of Thalidomide on melanoma tumor growth and blood supply patterns in C57 mice. METHODS: Thirty mice inoculated subcutaneously with B16F10 cells were randomly divided into the treatment group and the control group. Thalidomide was administered once a day at a dose of 200 mg/kg for the treatment group starting on the fifth day after inoculation, and an equivalent volume of 0.5% carboxylmethyl cellulose was administered similarly in the control group. The diameter of the tumors was measured daily after inoculation until the mice were sacrificed on the 19th day. The different blood supply patterns were counted after immunohistochemical and PAS histochemical double-Staining. VEGF, NF-kappaB, PCNA, MMP-2 and MMP-9 expression in tumor tissue was also assessed. RESULTS: The tumor volume(P = 0.019) and the number of vasculogenic mimicry(P = 0.03) and mosaic vessels(P = 0.004) in the treatment group were significantly decreased compared with the control group. VEGF(P = 0.004), NF-kappaB(P = 0.009), PCNA(P = 0.002), MMP-2 (P = 0.000), MMP-9(P = 0.002) protein expression and MMP-2(P = 0.000) and MMP-9(P = 0.000) mRNA in the treatment group were significantly lower than those in the control groups. CONCLUSION: Thalidomide inhibits vasculogenic mimicry channel and mosaic vessels formation in melanoma through the regulation of vasculogenic factors, and it can induce necrosis of melanoma cells, which may be related with the NF-kappaB signaling pathway.
Thalidomide reduces MPTP-induced decrease in striatal dopamine levels in mice.[Pubmed:9364513]
Neurosci Lett. 1997 Oct 3;234(2-3):123-6.
The effects of Thalidomide, a sedative, anti-inflammatory and immunosuppressive agent were studied in the MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) murine model of Parkinson's disease. The striatal levels of dopamine (DA) and of its main metabolites, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) were measured both in the MPTP control group (3 x 15 mg/kg intraperitoneally) and in the Thalidomide groups (repeated treatments at 25 mg/kg or 50 mg/kg postoperatively). For mice treated with Thalidomide, a dose-dependent protection was observed against the MPTP-induced decrease in DA. The decrease in HVA levels was totally antagonized by Thalidomide at both doses. That Thalidomide has activity in this model suggests that an inflammatory process may be involved in the induction of lesions by MPTP in DAergic neurons.
Thalidomide, a hypnotic with immune modulating properties, increases cataplexy in canine narcolepsy.[Pubmed:8905685]
Neuroreport. 1996 Aug 12;7(12):1881-6.
Thalidomide is a sedative hypnotic that was widely used in the 1950s but was withdrawn due to its teratogenic properties. The compound has recently been reintroduced as an immune modulating agent. Thalidomide significantly aggravates canine cataplexy, a pathological manifestation of rapid eye movement (RFM) sleep atonia seen in narcolepsy. This compound also increases REM sleep and slow wave sleep in these animals. In vitro receptor binding and enzyme assays demonstrate that Thalidomide does not bind to or enzymatically modulate the neurotransmitter systems reported to be involved in the regulation of cataplexy. Thalidomide may therefore affect cataplexy through its immune modulation properties. Further studies on the mechanisms of action of Thalidomide should increase our understanding of the pathophysiology of this disabling disorder.
Thalidomide inhibits the replication of human immunodeficiency virus type 1.[Pubmed:8327469]
Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5974-8.
Thalidomide, a selective inhibitor of tumor necrosis factor alpha (TNF-alpha) synthesis, suppresses the activation of latent human immunodeficiency virus type 1 (HIV-1) in a monocytoid (U1) line. The inhibition is dose dependent and occurs after exposure of the cells to recombinant TNF-alpha, phorbol myristate acetate, lipopolysaccharide, and other cytokine combinations. Associated with HIV-1 inhibition is a reduction in agonist-induced TNF-alpha protein and mRNA production. Thalidomide inhibition of virus replication in the phorbol myristate acetate- and recombinant TNF-alpha-stimulated T-cell line ACH-2 is not observed. The presence of Thalidomide also inhibits the activation of virus in the peripheral blood mononuclear cells of 16 out of 17 patients with advanced HIV-1 infection and AIDS. These results suggest the use of Thalidomide in a clinical setting to inhibit both virus replication and the TNF-alpha-induced systemic toxicity of HIV-1 and opportunistic infections.
Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes.[Pubmed:1997652]
J Exp Med. 1991 Mar 1;173(3):699-703.
Thalidomide selectively inhibits the production of human monocyte tumor necrosis factor alpha (TNF-alpha) when these cells are triggered with lipopolysaccharide and other agonists in culture. 40% inhibition occurs at the clinically achievable dose of the drug of 1 micrograms/ml. In contrast, the amount of total protein and individual proteins labeled with [35S]methionine and expressed on SDS-PAGE are not influenced. The amounts of interleukin 1 beta (IL-1 beta), IL-6, and granulocyte/macrophage colony-stimulating factor produced by monocytes remain unaltered. The selectivity of this drug may be useful in determining the role of TNF-alpha in vivo and modulating its toxic effects in a clinical setting.


