PI3Kγ inhibitor 1CAS# 1172118-03-4 |
- PF-04691502
Catalog No.:BCC3837
CAS No.:1013101-36-4
- A66
Catalog No.:BCC3715
CAS No.:1166227-08-2
- IC-87114
Catalog No.:BCC1161
CAS No.:371242-69-2
- PIK-93
Catalog No.:BCC2519
CAS No.:593960-11-3
- CAL-101 (Idelalisib, GS-1101)
Catalog No.:BCC1270
CAS No.:870281-82-6
- PIK-294
Catalog No.:BCC4995
CAS No.:900185-02-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1172118-03-4 | SDF | Download SDF |
| PubChem ID | 44123663 | Appearance | Powder |
| Formula | C32H26N8O2S | M.Wt | 586.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
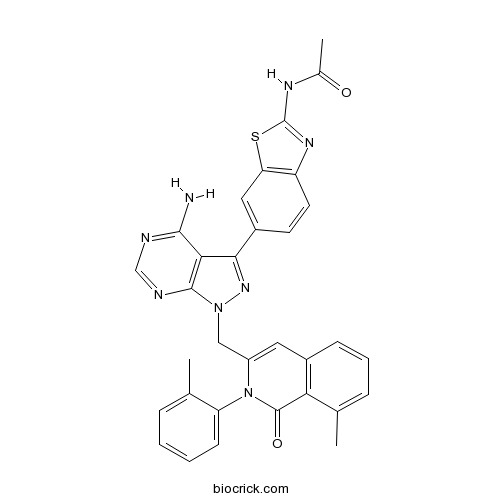
| Chemical Name | N-[6-[4-amino-1-[[8-methyl-2-(2-methylphenyl)-1-oxoisoquinolin-3-yl]methyl]pyrazolo[3,4-d]pyrimidin-3-yl]-1,3-benzothiazol-2-yl]acetamide | ||
| SMILES | CC1=CC=CC=C1N2C(=CC3=C(C2=O)C(=CC=C3)C)CN4C5=C(C(=N4)C6=CC7=C(C=C6)N=C(S7)NC(=O)C)C(=NC=N5)N | ||
| Standard InChIKey | UHZBJJRBPXOONG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C32H26N8O2S/c1-17-7-4-5-10-24(17)40-22(13-20-9-6-8-18(2)26(20)31(40)42)15-39-30-27(29(33)34-16-35-30)28(38-39)21-11-12-23-25(14-21)43-32(37-23)36-19(3)41/h4-14,16H,15H2,1-3H3,(H2,33,34,35)(H,36,37,41) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | PI3Kγ inhibitor 1 is a PI3Kδ and PI3Kγ inhibitor extracted from patent WO2014004470A1, Compound 168 in Table 4, has IC50s of <100 nM.In Vitro:In Table 3, PI3Kγ inhibitor 1 (Compound 168) inhibits PI3Kδ, PI3Kγ, PI3Kα and PI3Kβ with IC50s of <100 nM, <100 nM, <10 μM and <10 μM, respectively. PI3Kγ inhibitor 1 inhibits B cell proliferation with an EC50<100 nM. References: | |||||

PI3Kγ inhibitor 1 Dilution Calculator

PI3Kγ inhibitor 1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7045 mL | 8.5227 mL | 17.0454 mL | 34.0907 mL | 42.6134 mL |
| 5 mM | 0.3409 mL | 1.7045 mL | 3.4091 mL | 6.8181 mL | 8.5227 mL |
| 10 mM | 0.1705 mL | 0.8523 mL | 1.7045 mL | 3.4091 mL | 4.2613 mL |
| 50 mM | 0.0341 mL | 0.1705 mL | 0.3409 mL | 0.6818 mL | 0.8523 mL |
| 100 mM | 0.017 mL | 0.0852 mL | 0.1705 mL | 0.3409 mL | 0.4261 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PI3Kγ inhibitor 1 is a potent PI3Kγ inhibitor.
- M 1145
Catalog No.:BCC6053
CAS No.:1172089-00-7
- Sophoraisoflavone A
Catalog No.:BCN6826
CAS No.:117204-81-6
- Boc-Ala(3-pyridyl)-OH
Catalog No.:BCC3322
CAS No.:117142-26-4
- Fmoc-N-Me-Thr(tBu)-OH
Catalog No.:BCC3354
CAS No.:117106-20-4
- Fmoc-Thr(tBu)-OPfp
Catalog No.:BCC3553
CAS No.:117088-31-0
- Scutebarbatine C
Catalog No.:BCN2382
CAS No.:910099-75-1
- A 967079
Catalog No.:BCC7967
CAS No.:1170613-55-4
- 3'-Hydroxypuerarin
Catalog No.:BCN2816
CAS No.:117060-54-5
- Combretastatin A4
Catalog No.:BCC7089
CAS No.:117048-59-6
- Wulignan A1
Catalog No.:BCN5808
CAS No.:117047-76-4
- 3'-Methoxypuerarin
Catalog No.:BCN2900
CAS No.:117047-07-1
- Licopyranocoumarin
Catalog No.:BCN7900
CAS No.:117038-80-9
- HO-3867
Catalog No.:BCC5639
CAS No.:1172133-28-6
- Taxacin
Catalog No.:BCN6950
CAS No.:117229-54-6
- Soyasaponin Aa
Catalog No.:BCN2597
CAS No.:117230-33-8
- 8alpha-Hydroxylabda-13(16),14-dien-19-yl p-hydroxycinnamate
Catalog No.:BCN1609
CAS No.:117254-98-5
- gamma-secretase modulator 1
Catalog No.:BCC1583
CAS No.:1172637-87-4
- RS 102895 hydrochloride
Catalog No.:BCC7260
CAS No.:1173022-16-6
- STO-609 acetate
Catalog No.:BCC7112
CAS No.:1173022-21-3
- GW 583340 dihydrochloride
Catalog No.:BCC7300
CAS No.:1173023-85-2
- U0126-EtOH
Catalog No.:BCC1066
CAS No.:1173097-76-1
- PKI-402
Catalog No.:BCC3843
CAS No.:1173204-81-3
- Clocinnamox mesylate
Catalog No.:BCC5684
CAS No.:117332-69-1
- Emeheterone
Catalog No.:BCN7285
CAS No.:117333-12-7
PI3Kgamma Deficient NOD-Mice Are Protected from Diabetes by Restoring the Balance of Regulatory to Effector-T-Cells.[Pubmed:28081180]
PLoS One. 2017 Jan 12;12(1):e0169695.
With a steady increase in its incidence and lack of curative treatment, type 1 diabetes (T1D) has emerged as a major health problem worldwide. To design novel effective therapies, there is a pressing need to identify regulatory targets controlling the balance of autoreactive to regulatory-T-cells (Tregs). We previously showed that the inhibition of the gamma-subunit of the Phosphoinositide-3-kinase (PI3K), significantly suppress autoimmune-diabetes. To further delineate the mechanisms and the selectivity of specific immune modulation by PI3Kgamma-inhibition, we developed a new NOD mouse model of T1D lacking the gamma-subunit of PI3K. Strikingly, the loss of PI3Kgamma protected 92% of the NOD-mice from developing spontaneous diabetes. The NOD.PI3Kgamma-/- mice are protected from insulitis secondary to a defect in CD4 and CD8 autoreactive-T-cells activation and survival. In addition, PI3Kgamma-deficiency promoted Treg generation in-vitro and in-vivo. Furthermore, PI3Kgamma-inhibitor (AS605240) inhibited proliferation and cytokine production of a human CD4+ T-cell clone specific for GAD555-567 peptide that was isolated from a patient with T1D. These studies demonstrate the key role of the PI3Kgamma pathway in regulating autoimmune-diabetes and provide rationales for future devise of anti- PI3Kgamma therapy in T1D.
SAR study of 5-alkynyl substituted quinazolin-4(3H)-ones as phosphoinositide 3-kinase delta (PI3Kdelta) inhibitors.[Pubmed:27846451]
Eur J Med Chem. 2017 Jan 5;125:1156-1171.
PI3Kdelta is a key component in the aberrant signaling transduction in B cell malignancy, therefore specific targeting PI3Kdelta has become an attractive molecularly targeted therapy for chronic lymphocytic leukemia (CLL). Herein, we describe the discovery and optimization of a series of 5-alkynyl substituted PI3Kdelta inhibitors based on the first FDA-approved inhibitor idelalisib. Compound 8d bearing the 1-morpholinohex-5-yn-1-one moiety as the C5-substituent was identified to have high potency against PI3Kdelta (3.82 nM) and SU-DHL-6 cells (7.60 nM), respectively. It was 154-fold selective over PI3Kalpha, 133-fold selective against PI3Kbeta, and 24-fold selective against PI3Kgamma. Treatment of MOLT-4 and SU-DHL-6 cells with compound 8d for 1 h resulted in reduction of phosphorylation of both Akt (S473) and its downstream S6k1 (T389) in a concentration-dependent manner. Compound 8d showed potent anti-proliferative activity as well against T lymphoblast MOLT-4, suggesting its potential activity in T-cell leukemia.
Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells.[Pubmed:27828943]
Nature. 2016 Nov 17;539(7629):443-447.
Recent clinical trials using immunotherapy have demonstrated its potential to control cancer by disinhibiting the immune system. Immune checkpoint blocking (ICB) antibodies against cytotoxic-T-lymphocyte-associated protein 4 or programmed cell death protein 1/programmed death-ligand 1 have displayed durable clinical responses in various cancers. Although these new immunotherapies have had a notable effect on cancer treatment, multiple mechanisms of immune resistance exist in tumours. Among the key mechanisms, myeloid cells have a major role in limiting effective tumour immunity. Growing evidence suggests that high infiltration of immune-suppressive myeloid cells correlates with poor prognosis and ICB resistance. These observations suggest a need for a precision medicine approach in which the design of the immunotherapeutic combination is modified on the basis of the tumour immune landscape to overcome such resistance mechanisms. Here we employ a pre-clinical mouse model system and show that resistance to ICB is directly mediated by the suppressive activity of infiltrating myeloid cells in various tumours. Furthermore, selective pharmacologic targeting of the gamma isoform of phosphoinositide 3-kinase (PI3Kgamma), highly expressed in myeloid cells, restores sensitivity to ICB. We demonstrate that targeting PI3Kgamma with a selective inhibitor, currently being evaluated in a phase 1 clinical trial (NCT02637531), can reshape the tumour immune microenvironment and promote cytotoxic-T-cell-mediated tumour regression without targeting cancer cells directly. Our results introduce opportunities for new combination strategies using a selective small molecule PI3Kgamma inhibitor, such as IPI-549, to overcome resistance to ICB in patients with high levels of suppressive myeloid cell infiltration in tumours.
alpha-Tocopheryl Phosphate Induces VEGF Expression via CD36/PI3Kgamma in THP-1 Monocytes.[Pubmed:28059487]
J Cell Biochem. 2017 Jul;118(7):1855-1867.
The CD36 scavenger receptor binds several ligands and mediates ligand uptake and ligand-dependent signal transduction and gene expression, events that may involve CD36 internalization. Here we show that CD36 internalization in THP-1 monocytes is triggered by alpha-tocopherol (alphaT) and more strongly by alpha-tocopheryl phosphate (alphaTP) and EPC-K1, a phosphate diester of alphaTP and L-ascorbic acid. alphaTP-triggered CD36 internalization is prevented by the specific covalent inhibitor of selective lipid transport by CD36, sulfo-N-succinimidyl oleate (SSO). Moreover, SSO inhibited the CD36-mediated uptake of 14C-labelled alphaTP suggesting that alphaTP binding and internalization of CD36 is involved in cellular alphaTP uptake, whereas the uptake of alphaT was less affected. Similar to that, inhibition of selective lipid transport of the SR-BI scavenger receptor resulted mainly in reduction of alphaTP and not alphaT uptake. In contrast, uptake of alphaT was mainly inhibited by Dynasore, an inhibitor of clathrin-mediated endocytosis, suggesting that the differential regulatory effects of alphaTP and alphaT on signaling may be influenced by their different routes of uptake. Interestingly, alphaTP and EPC-K1 also reduced the neutral lipid content of THP-1 cells and the phagocytosis of fluorescent Staphylococcus aureus bioparticles. Moreover, induction of the vascular endothelial growth factor (VEGF) promoter activity by alphaTP occurred via CD36/PI3Kgamma/Akt, as it could be inhibited by specific inhibitors of this pathway (SSO, Wortmannin, AS-605240). These results suggest that alphaTP activates PI3Kgamma/Akt signaling leading to VEGF expression in monocytes after binding to and/or transport by CD36, a receptor known to modulate angiogenesis in response to amyloid beta, oxLDL, and thrombospondin. J. Cell. Biochem. 118: 1855-1867, 2017. (c) 2017 Wiley Periodicals, Inc.
PI3Kgamma is a molecular switch that controls immune suppression.[Pubmed:27642729]
Nature. 2016 Nov 17;539(7629):437-442.
Macrophages play critical, but opposite, roles in acute and chronic inflammation and cancer. In response to pathogens or injury, inflammatory macrophages express cytokines that stimulate cytotoxic T cells, whereas macrophages in neoplastic and parasitic diseases express anti-inflammatory cytokines that induce immune suppression and may promote resistance to T cell checkpoint inhibitors. Here we show that macrophage PI 3-kinase gamma controls a critical switch between immune stimulation and suppression during inflammation and cancer. PI3Kgamma signalling through Akt and mTor inhibits NFkappaB activation while stimulating C/EBPbeta activation, thereby inducing a transcriptional program that promotes immune suppression during inflammation and tumour growth. By contrast, selective inactivation of macrophage PI3Kgamma stimulates and prolongs NFkappaB activation and inhibits C/EBPbeta activation, thus promoting an immunostimulatory transcriptional program that restores CD8(+) T cell activation and cytotoxicity. PI3Kgamma synergizes with checkpoint inhibitor therapy to promote tumour regression and increased survival in mouse models of cancer. In addition, PI3Kgamma-directed, anti-inflammatory gene expression can predict survival probability in cancer patients. Our work thus demonstrates that therapeutic targeting of intracellular signalling pathways that regulate the switch between macrophage polarization states can control immune suppression in cancer and other disorders.


