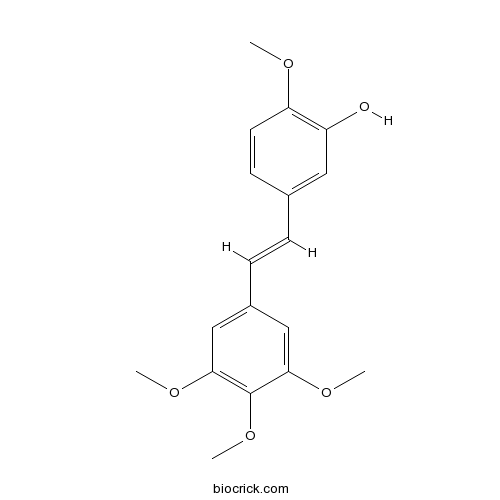
Combretastatin A4Tubulin polymerization inhibitor. Antitumor, antiangiogenic and antimetastatic CAS# 117048-59-6 |
- Fosbretabulin (Combretastatin A4 Phosphate (CA4P)) Disodium
Catalog No.:BCC4600
CAS No.:168555-66-6
- Eribulin
Catalog No.:BCC5174
CAS No.:253128-41-5
- Eribulin mesylate
Catalog No.:BCC5173
CAS No.:441045-17-6
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 117048-59-6 | SDF | Download SDF |
| PubChem ID | 5386397 | Appearance | Powder |
| Formula | C18H20O5 | M.Wt | 316.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CRC 87-09, NSC 817373 | ||
| Solubility | DMSO : 100 mg/mL (316.11 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 2-methoxy-5-[(E)-2-(3,4,5-trimethoxyphenyl)ethenyl]phenol | ||
| SMILES | COC1=C(C=C(C=C1)C=CC2=CC(=C(C(=C2)OC)OC)OC)O | ||
| Standard InChIKey | HVXBOLULGPECHP-AATRIKPKSA-N | ||
| Standard InChI | InChI=1S/C18H20O5/c1-20-15-8-7-12(9-14(15)19)5-6-13-10-16(21-2)18(23-4)17(11-13)22-3/h5-11,19H,1-4H3/b6-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antitumor, antiangiogenic and antimetastatic agent, in vitro and in vivo. Inhibits tubulin polymerization. |

Combretastatin A4 Dilution Calculator

Combretastatin A4 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1611 mL | 15.8053 mL | 31.6106 mL | 63.2211 mL | 79.0264 mL |
| 5 mM | 0.6322 mL | 3.1611 mL | 6.3221 mL | 12.6442 mL | 15.8053 mL |
| 10 mM | 0.3161 mL | 1.5805 mL | 3.1611 mL | 6.3221 mL | 7.9026 mL |
| 50 mM | 0.0632 mL | 0.3161 mL | 0.6322 mL | 1.2644 mL | 1.5805 mL |
| 100 mM | 0.0316 mL | 0.1581 mL | 0.3161 mL | 0.6322 mL | 0.7903 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Wulignan A1
Catalog No.:BCN5808
CAS No.:117047-76-4
- 3'-Methoxypuerarin
Catalog No.:BCN2900
CAS No.:117047-07-1
- Licopyranocoumarin
Catalog No.:BCN7900
CAS No.:117038-80-9
- Anwuweizonic acid
Catalog No.:BCN3633
CAS No.:117020-59-4
- Boc-Asp(Ofm)-OH
Catalog No.:BCC3366
CAS No.:117014-32-1
- Bis(2-ethylhexyl) phthalate
Catalog No.:BCN6054
CAS No.:117-81-7
- 2-Anthraquinonecarboxylic acid
Catalog No.:BCN3451
CAS No.:117-78-2
- 2-Amino-3-hydroxyanthraquinone
Catalog No.:BCC8527
CAS No.:117-77-1
- Quercetin
Catalog No.:BCN6049
CAS No.:117-39-5
- Dantron
Catalog No.:BCN6048
CAS No.:117-10-2
- Rubiadin
Catalog No.:BCN6047
CAS No.:117-02-2
- Sculponeatic acid
Catalog No.:BCN6046
CAS No.:1169806-02-3
- 3'-Hydroxypuerarin
Catalog No.:BCN2816
CAS No.:117060-54-5
- A 967079
Catalog No.:BCC7967
CAS No.:1170613-55-4
- Scutebarbatine C
Catalog No.:BCN2382
CAS No.:910099-75-1
- Fmoc-Thr(tBu)-OPfp
Catalog No.:BCC3553
CAS No.:117088-31-0
- Fmoc-N-Me-Thr(tBu)-OH
Catalog No.:BCC3354
CAS No.:117106-20-4
- Boc-Ala(3-pyridyl)-OH
Catalog No.:BCC3322
CAS No.:117142-26-4
- Sophoraisoflavone A
Catalog No.:BCN6826
CAS No.:117204-81-6
- M 1145
Catalog No.:BCC6053
CAS No.:1172089-00-7
- PI3Kγ inhibitor 1
Catalog No.:BCC4180
CAS No.:1172118-03-4
- HO-3867
Catalog No.:BCC5639
CAS No.:1172133-28-6
- Taxacin
Catalog No.:BCN6950
CAS No.:117229-54-6
- Soyasaponin Aa
Catalog No.:BCN2597
CAS No.:117230-33-8
Combretastatin A4-beta-Galactosyl Conjugates for Ovarian Cancer Prodrug Monotherapy.[Pubmed:28197314]
ACS Med Chem Lett. 2017 Jan 20;8(2):211-214.
Chemotherapy for ovarian cancer often causes severe side effects. As candidates for Combretastatin A4 (CA4) prodrug for ovarian cancer prodrug monotherapy (PMT), we designed and synthesized two beta-galactose-conjugated CA4s (CA4-betaGals), CA4-betaGal-1 and CA4-betaGal-2. CA4 was liberated from CA4-betaGals by beta-galactosidase, an enzyme more strongly expressed in ovarian cancer cells than normal cells. CA4-betaGal-2, which has a self-immolative benzyl linker between CA4 and the beta-galactose moiety, was more cytotoxic to ovarian cancer cell lines than CA4-betaGal-1 without a linker. Therefore, CA4-betaGal-2 can serve as a platform for the design and manufacture of prodrugs for ovarian cancer PMT.
A poly(l-glutamic acid)-combretastatin A4 conjugate for solid tumor therapy: Markedly improved therapeutic efficiency through its low tissue penetration in solid tumor.[Pubmed:28167300]
Acta Biomater. 2017 Apr 15;53:179-189.
Combretastatin A4 (CA4) is a leading agent in vascular disrupting strategies for tumor therapy. Although many small-molecule prodrugs of CA4 have been developed to improve its solubility, the overall therapeutic efficiency is moderate. A key reason for this is the reversible effect that CA4 has on tubulin as well as its rapid clearance from plasma and tissues. In this study, we proposed a poly(l-glutamic acid)-CA4 conjugate (PLG-CA4) nanomedicine to fulfill the requirements for fully liberating the potential of CA4 on tumor therapy. Enhanced accumulation and retention of CA4 in tumor tissue, especially, high distribution and gradual release around tumor blood vessels resulted in prolonged vascular disruption and markedly enhanced therapeutic efficiency. We examined and compared the therapeutic effect of PLG-CA4 and commercial combretastatin-A4 phosphate (CA4P) in a murine colon C26 tumor. PLG-CA4 showed significantly prolonged retention in plasma and tumor tissue. Most importantly, the PLG-CA4 was mainly distributed around the tumor vessels because of its low tissue penetration in solid tumor. Pathology tests showed that PLG-CA4 treatment resulted in persistent vascular disruption and tumor damage 72h after a single injection, this in contrast to CA4P treatment, which showed quick relapse at an equal dose. Tumor suppression tests showed that PLG-CA4 treatment resulted in a tumor suppression rate of 74%, which indicates a significant advantage when compared to tumor suppression rate of the CA4P group, which was 24%. This is the first time that an advantage of the polymeric CA4 nanomedicine with low tissue penetration for solid tumor therapy has been shown. Thus, the results presented in this study provide a new idea for enhancing the tumor therapeutic effect of vascular disrupting agents. STATEMENT OF SIGNIFICANCE: Nanomedicine usually has low tissue penetration in solid tumors, which limits the efficacy of nanomedicine in most cases. But herein, we demonstrate a nanosized vascular disruptive agent (VDA) PLG-CA4 has supper advantages over small molecular combretastatin-A4 phosphate (CA4P) because the PLG-CA4 was mainly distributed around the tumor vessels due to its low tissue penetration in solid tumor.
The Blood Flow Shutdown Induced by Combretastatin A4 Impairs Gemcitabine Delivery in a Mouse Hepatocarcinoma.[Pubmed:28066252]
Front Pharmacol. 2016 Dec 23;7:506.
In recent clinical studies, vascular disrupting agents (VDAs) are mainly used in combination with chemotherapy. However, an often overlooked concern in treatment combination is the VDA-induced impairment of chemotherapy distribution in the tumor. The work presented here investigated the impact of blood flow shutdown induced by Combretastatin A4 (CA4) on gemcitabine uptake into mouse hepatocarcinoma. At 2 h after CA4 treatment, using DCE-MRI, a significant decrease in the perfusion-relevant parameters K(trans) and Vp were observed in treated group compared with the control group. The blood flow shutdown was indeed confirmed by a histology study. In a third experiment, the total gemcitabine uptake was found to be significantly lower in treated tumors, as assessed in a separate experiment using ex vivo fluorine nuclear magnetic resonance spectroscopy. The amount of active metabolite gemcitabine triphosphate was also lower in treated tumors. In conclusion, the blood flow shutdown induced by VDAs can impact negatively on the delivery of small cytotoxic agents in tumors. The present study outlines the importance of monitoring the tumor vascular function when designing drug combinations.
Structurally simplified biphenyl combretastatin A4 derivatives retain in vitro anti-cancer activity dependent on mitotic arrest.[Pubmed:28253265]
PLoS One. 2017 Mar 2;12(3):e0171806.
The cis-stilbene, Combretastatin A4 (CA4), is a potent microtubule targeting and vascular damaging agent. Despite promising results at the pre-clinical level and extensive clinical evaluation, CA4 has yet to be approved for therapeutic use. One impediment to the development of CA4 is an inherent conformational instability about the ethylene linker, which joins two aromatic rings. We have previously published preliminary data regarding structurally simplified biphenyl derivatives of CA4, lacking an ethylene linker, which retain anti-proliferative and pro-apoptotic activity, albeit at higher doses. Our current study provides a more comprehensive evaluation regarding the anti-proliferative and pro-apoptotic properties of biphenyl CA4 derivatives in both 2D and 3D cancerous and non-cancerous cell models. Computational analysis has revealed that cytotoxicity of CA4 and biphenyl analogues correlates with predicted tubulin affinity. Additional mechanistic evaluation of the biphenyl derivatives found that their anti-cancer activity is dependent on prolonged mitotic arrest, in a similar manner to CA4. Lastly, we have shown that cancer cells deficient in the extrinsic pathway of apoptosis experience delayed cell death following treatment with CA4 or analogues. Biphenyl derivatives of CA4 represent structurally simplified analogues of CA4, which retain a similar mechanism of action. The biphenyl analogues warrant in vivo examination to evaluate their potential as vascular damaging agents.
Combretastatin A4-induced differential cytotoxicity and reduced metastatic ability by inhibition of AKT function in human gastric cancer cells.[Pubmed:17646428]
J Pharmacol Exp Ther. 2007 Oct;323(1):365-73.
Combretastatin A4 (CA4) is a drug that targets tumor vasculature to inhibit angiogenesis. Whether CA4 has a direct effect on gastric cancer is not known. We herein investigated the effect of CA4 on growth and metastasis of gastric cancer cells at clinically achievable concentration and explored the associated antitumor mechanisms. Nine human gastric cancer cell lines, including two metastatic gastric cancer cell lines (AGS-GFPM1/2), constitutively expressing green fluorescence protein (GFP) were used. These metastatic AGS-GFPM1/2 cells expressed a higher level of phosphorylated serine 473 on AKT (p-AKT). Our results showed that CA4 (0.02-20 microM) has significant in vitro effects on reducing cell attachment, migration, invasiveness, as well as cell cycle G2/M disturbance on p-AKT-positive gastric cancer cells. In addition, a phosphoinositide 3-kinase inhibitor, LY294002 [2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride], a specific AKT inhibitor, and 0.2 to 20 microM CA4 displayed a similar response profile on p-AKT-positive cells, suggesting that CA4-induced effect was mediated by inhibition of the PI3 kinase/AKT pathway. The results from in vivo GFP monitoring system indicated that CA4 phosphate (CA4-P; 200 mg/kg) significantly inhibited the s.c. and intra-abdominal growth of xenotransplanted AGS-GFPM2 cells in nude mice. Furthermore, CA4-P treatment showed a remarkable ability to inhibit gastric tumor metastasis as well as attenuate p-AKT expression. In conclusion, our study is the first to find that CA4 inhibited AKT activity in human gastric cancer cells. The decreased AKT activity correlated well with the CA4 antitumor growth response and decrease of metastasis. Further investigation on drugs targeting the PI3 kinase-AKT pathway may provide a new approach for the treatment of human gastric cancer.
Potent anti-metastatic activity of combretastatin-A4.[Pubmed:11562761]
Int J Oncol. 2001 Oct;19(4):821-5.
The requirement for tumour vascularisation to permit the expansion of solid tumours beyond a threshold size of approximately 1 mm diameter has focussed attention on anti-vascular and anti-angiogenic agents for cancer therapy. Combretastatin-A4 (cis CA-4P) is a tubulin-binding agent that is cytotoxic for proliferating endothelial cells in vitro and causes anti-vascular effects in the established tumour vessels of some primary tumours. Preliminary data from Phase I clinical trials indicate that cis CA-4 may also be effective in targeting the vasculature of human tumours. As metastatic disease is the principal cause of mortality in cancer, we have investigated the effects of cis CA-4 on metastatic development using an in vivo model. We show that bolus or continuous administration of cis CA-4P results in potent inhibition of metastases derived from ectopic primary Lewis lung carcinomas in mice whereas the trans CA-4 isomer is without effect. These data further characterise the activity of CA-4 in vivo and suggest that the drug should be evaluated clinically as an anti-metastatic agent.
Antitumor activity of combretastatin-A4 phosphate, a natural product tubulin inhibitor.[Pubmed:8913833]
Invest New Drugs. 1996;14(2):131-7.
The tubulin-binding natural product combretastatin A-4 (CA-4) was tested for antitumor activity against fresh human tumors in vitro and 2 mouse tumors, both in vitro and in vivo. In colony forming assays using 10% fetal bovine serum, CA-4 was inhibitory in 27/40 human ovary cancers with a mean IC50 of 3.18 micrograms/mL for a 1-hour exposure (n = 35 specimens) and 0.27 microgramf1p4for a continuous exposure to CA-4 for 11-14 days (n = 5 specimens). Murine B-16 melanoma and P-388 leukemia were also highly sensitive to CA-4 in vitro with an identical IC50 value of 0.0007 micrograms/mL for continuous drug exposure for 8 days. Comparable in vitro cell culture studies performed in serum concentrations higher than 10%, revealed a significant loss of cytotoxic potency. Using the same reversed-phase HPLC technique as developed for paclitaxel, CA-4 was shown to bind to serum proteins (> or = 30,000 mw) > 99% and to albumin approximately 70%. CA-4 was only marginally active (25% increased lifespan) in DBA/2 mice bearing P-388 leukemia who were given doses of 100 mg/kg IP on either days, 1, 5 and 9 (p = 0.075 by Wilcoxon analysis) or on consecutive days 1-9 (p = 0.19 compared to control). A higher IP dose of 150 mg/kg on days 1, 5 and 9 did not delay subcutaneous B-16 melanoma tumor growth in C57/B1 mice. These findings demonstrate a substantial loss of antitumor efficacy for CA-4 in physiologic serum concentrations in vitro. No consistent antitumor activity was observed in two murine tumor models in vivo.


