EmeheteroneCAS# 117333-12-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

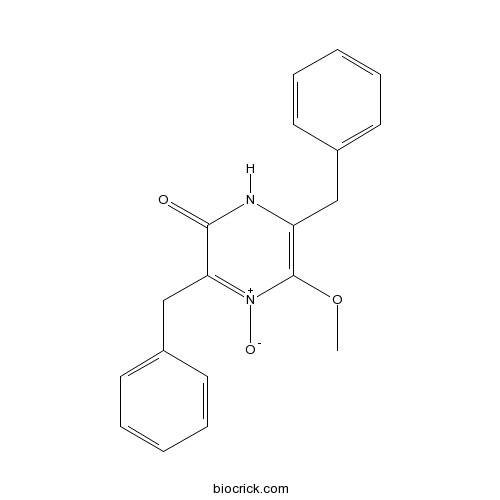
| Cas No. | 117333-12-7 | SDF | Download SDF |
| PubChem ID | 14898343 | Appearance | Powder |
| Formula | C19H18N2O3 | M.Wt | 322.36 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,6-dibenzyl-5-methoxy-4-oxido-1H-pyrazin-4-ium-2-one | ||
| SMILES | COC1=C(NC(=O)C(=[N+]1[O-])CC2=CC=CC=C2)CC3=CC=CC=C3 | ||
| Standard InChIKey | JXYUVGGSKHTJHO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H18N2O3/c1-24-19-16(12-14-8-4-2-5-9-14)20-18(22)17(21(19)23)13-15-10-6-3-7-11-15/h2-11H,12-13H2,1H3,(H,20,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Emeheterone is a natural product from the fresh fruiting bodies of the basidiomycetes Albatrellus confluens. |
| Structure Identification | Helvetica Chimica Acta, 2001, 84(1):259-262.Albaconol, A Novel Prenylated Resorcinol (=Benzene1,3-diol) from BasidiomycetesAlbatrellus confluens[Reference: WebLink]
|

Emeheterone Dilution Calculator

Emeheterone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1021 mL | 15.5106 mL | 31.0212 mL | 62.0424 mL | 77.553 mL |
| 5 mM | 0.6204 mL | 3.1021 mL | 6.2042 mL | 12.4085 mL | 15.5106 mL |
| 10 mM | 0.3102 mL | 1.5511 mL | 3.1021 mL | 6.2042 mL | 7.7553 mL |
| 50 mM | 0.062 mL | 0.3102 mL | 0.6204 mL | 1.2408 mL | 1.5511 mL |
| 100 mM | 0.031 mL | 0.1551 mL | 0.3102 mL | 0.6204 mL | 0.7755 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Clocinnamox mesylate
Catalog No.:BCC5684
CAS No.:117332-69-1
- PKI-402
Catalog No.:BCC3843
CAS No.:1173204-81-3
- U0126-EtOH
Catalog No.:BCC1066
CAS No.:1173097-76-1
- GW 583340 dihydrochloride
Catalog No.:BCC7300
CAS No.:1173023-85-2
- STO-609 acetate
Catalog No.:BCC7112
CAS No.:1173022-21-3
- RS 102895 hydrochloride
Catalog No.:BCC7260
CAS No.:1173022-16-6
- gamma-secretase modulator 1
Catalog No.:BCC1583
CAS No.:1172637-87-4
- 8alpha-Hydroxylabda-13(16),14-dien-19-yl p-hydroxycinnamate
Catalog No.:BCN1609
CAS No.:117254-98-5
- Soyasaponin Aa
Catalog No.:BCN2597
CAS No.:117230-33-8
- Taxacin
Catalog No.:BCN6950
CAS No.:117229-54-6
- HO-3867
Catalog No.:BCC5639
CAS No.:1172133-28-6
- PI3Kγ inhibitor 1
Catalog No.:BCC4180
CAS No.:1172118-03-4
- 9,9-Bis[4-(2-hydroxyethoxy)phenyl]fluorene
Catalog No.:BCC8796
CAS No.:117344-32-8
- 2-Hydroxysaclofen
Catalog No.:BCC6579
CAS No.:117354-64-0
- AZD6482
Catalog No.:BCC2523
CAS No.:1173900-33-8
- Endothelin 3 (human, rat)
Catalog No.:BCC5713
CAS No.:117399-93-6
- Artoheterophyllin B
Catalog No.:BCN6050
CAS No.:1174017-37-8
- Dimethylwulignan A1
Catalog No.:BCN3624
CAS No.:117404-43-0
- AZD2461
Catalog No.:BCC2214
CAS No.:1174043-16-3
- 4-O-beta-Glucopyranosyl-cis-coumaric acid
Catalog No.:BCN1608
CAS No.:117405-48-8
- D-CPP-ene
Catalog No.:BCC6999
CAS No.:117414-74-1
- 2-Methyl-6-(p-tolyl)heptane-2,3-diol
Catalog No.:BCN7249
CAS No.:117421-22-4
- Xenin 8
Catalog No.:BCC5876
CAS No.:117442-28-1
- Wilforol E
Catalog No.:BCN8058
CAS No.:117456-86-7
New taxa in Aspergillus section Usti.[Pubmed:21892244]
Stud Mycol. 2011 Jun 30;69(1):81-97.
Based on phylogenetic analysis of sequence data, Aspergillus section Usti includes 21 species, inclucing two teleomorphic species Aspergillus heterothallicus (= Emericella heterothallica) and Fennellia monodii. Aspergillus germanicus sp. nov. was isolated from indoor air in Germany. This species has identical ITS sequences with A. insuetusCBS 119.27, but is clearly distinct from that species based on beta-tubulin and calmodulin sequence data. This species is unable to grow at 37 degrees C, similarly to A. keveii and A. insuetus. Aspergillus carlsbadensis sp. nov. was isolated from the Carlsbad Caverns National Park in New Mexico. This taxon is related to, but distinct from a clade including A. calidoustus, A. pseudodeflectus, A. insuetus and A. keveii on all trees. This species is also unable to grow at 37 degrees C, and acid production was not observed on CREA. Aspergillus californicus sp. nov. is proposed for an isolate from chamise chaparral (Adenostoma fasciculatum) in California. It is related to a clade including A. subsessilis and A. kassunensis on all trees. This species grew well at 37 degrees C, and acid production was not observed on CREA. The strain CBS 504.65 from soil in Turkey showed to be clearly distinct from the A. deflectus ex-type strain, indicating that this isolate represents a distinct species in this section. We propose the name A. turkensis sp. nov. for this taxon. This species grew, although rather restrictedly at 37 degrees C, and acid production was not observed on CREA. Isolates from stored maize, South Africa, as a culture contaminant of Bipolaris sorokiniana from indoor air in Finland proved to be related to, but different from A. ustus and A. puniceus. The taxon is proposed as the new species A. pseudoustus. Although supported only by low bootstrap values, F. monodii was found to belong to section Usti based on phylogenetic analysis of either loci BLAST searches to the GenBank database also resulted in closest hits from section Usti. This species obviously does not belong to the Fennellia genus, instead it is a member of the Emericella genus. However, in accordance with the guidelines of the Amsterdam Declaration on fungal nomenclature (Hawksworth et al. 2011), and based on phylogenetic and physiological evidence, we propose the new combination Aspergillus monodii comb. nov. for this taxon. Species assigned to section Usti can be assigned to three chemical groups based on the extrolites. Aspergillus ustus, A. granulosus and A. puniceus produced ustic acid, while A. ustus and A. puniceus also produced austocystins and versicolorins. In the second chemical group, A. pseudodeflectus produced drimans in common with the other species in this group, and also several unique unknown compounds. Aspergillus calidoustus isolates produced drimans and ophiobolins in common with A. insuetus and A. keveii, but also produced austins. Aspergillus insuetus isolates also produced pergillin while A. keveii isolates produced nidulol. In the third chemical group, E. heterothallica has been reported to produce emethallicins, 5'-hydroxyaveranthin, Emeheterone, emesterones, 5'-hydroxyaveranthin.


