Endothelin 3 (human, rat)Potent vasoconstrictor CAS# 117399-93-6 |
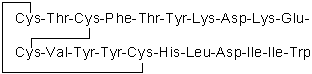
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 117399-93-6 | SDF | Download SDF |
| PubChem ID | 16132998 | Appearance | Powder |
| Formula | C121H168N26O33S4 | M.Wt | 2643 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ET-3 | ||
| Solubility | H2O Peptide Solubility and Storage Guidelines: 1. Calculate the length of the peptide. 2. Calculate the overall charge of the entire peptide according to the following table: 3. Recommended solution: | ||
| Sequence | CTCFTYKDKECVYYCHLDIIW (Modifications: Disulfide bridge between 1 - 15, 3 - 11) | ||
| SMILES | CCC(C)C(C(=O)NC(C(C)CC)C(=O)NC(CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)C(CC(=O)O)NC(=O)C(CC(C)C)NC(=O)C(CC3=CN=CN3)NC(=O)C4CSSCC(C(=O)NC(C(=O)NC5CSSCC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N4)CC6=CC=C(C=C6)O)CC7=CC=C(C=C7)O)C(C)C)NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC5=O)CC8=CC=CC=C8)C(C)O)CC9=CC=C(C=C9)O)CCCCN)CC(=O)O)CCCCN)CCC(=O)O)C(C)O)N | ||
| Standard InChIKey | OQGZWNZGVYLIFX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C121H168N26O33S4/c1-11-62(7)97(117(175)139-89(121(179)180)49-70-53-126-77-25-17-16-24-75(70)77)145-118(176)98(63(8)12-2)144-112(170)88(52-95(157)158)136-105(163)81(44-60(3)4)131-109(167)86(50-71-54-125-59-127-71)134-113(171)90-56-182-181-55-76(124)101(159)146-99(64(9)148)120(178)142-91-57-183-184-58-92(115(173)143-96(61(5)6)116(174)137-84(48-69-32-38-74(152)39-33-69)107(165)132-82(108(166)141-90)46-67-28-34-72(150)35-29-67)140-104(162)80(40-41-93(153)154)130-102(160)78(26-18-20-42-122)129-110(168)87(51-94(155)156)135-103(161)79(27-19-21-43-123)128-106(164)83(47-68-30-36-73(151)37-31-68)138-119(177)100(65(10)149)147-111(169)85(133-114(91)172)45-66-22-14-13-15-23-66/h13-17,22-25,28-39,53-54,59-65,76,78-92,96-100,126,148-152H,11-12,18-21,26-27,40-52,55-58,122-124H2,1-10H3,(H,125,127)(H,128,164)(H,129,168)(H,130,160)(H,131,167)(H,132,165)(H,133,172)(H,134,171)(H,135,161)(H,136,163)(H,137,174)(H,138,177)(H,139,175)(H,140,162)(H,141,166)(H,142,178)(H,143,173)(H,144,170)(H,145,176)(H,146,159)(H,147,169)(H,153,154)(H,155,156)(H,157,158)(H,179,180) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous neuropeptide and potent vasoconstrictor. Displays selectivity for the putative ETC endothelin receptor. |

Endothelin 3 (human, rat) Dilution Calculator

Endothelin 3 (human, rat) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- AZD6482
Catalog No.:BCC2523
CAS No.:1173900-33-8
- 2-Hydroxysaclofen
Catalog No.:BCC6579
CAS No.:117354-64-0
- 9,9-Bis[4-(2-hydroxyethoxy)phenyl]fluorene
Catalog No.:BCC8796
CAS No.:117344-32-8
- Emeheterone
Catalog No.:BCN7285
CAS No.:117333-12-7
- Clocinnamox mesylate
Catalog No.:BCC5684
CAS No.:117332-69-1
- PKI-402
Catalog No.:BCC3843
CAS No.:1173204-81-3
- U0126-EtOH
Catalog No.:BCC1066
CAS No.:1173097-76-1
- GW 583340 dihydrochloride
Catalog No.:BCC7300
CAS No.:1173023-85-2
- STO-609 acetate
Catalog No.:BCC7112
CAS No.:1173022-21-3
- RS 102895 hydrochloride
Catalog No.:BCC7260
CAS No.:1173022-16-6
- gamma-secretase modulator 1
Catalog No.:BCC1583
CAS No.:1172637-87-4
- 8alpha-Hydroxylabda-13(16),14-dien-19-yl p-hydroxycinnamate
Catalog No.:BCN1609
CAS No.:117254-98-5
- Artoheterophyllin B
Catalog No.:BCN6050
CAS No.:1174017-37-8
- Dimethylwulignan A1
Catalog No.:BCN3624
CAS No.:117404-43-0
- AZD2461
Catalog No.:BCC2214
CAS No.:1174043-16-3
- 4-O-beta-Glucopyranosyl-cis-coumaric acid
Catalog No.:BCN1608
CAS No.:117405-48-8
- D-CPP-ene
Catalog No.:BCC6999
CAS No.:117414-74-1
- 2-Methyl-6-(p-tolyl)heptane-2,3-diol
Catalog No.:BCN7249
CAS No.:117421-22-4
- Xenin 8
Catalog No.:BCC5876
CAS No.:117442-28-1
- Wilforol E
Catalog No.:BCN8058
CAS No.:117456-86-7
- Triptonodiol
Catalog No.:BCN6782
CAS No.:117456-87-8
- BCECF-AM
Catalog No.:BCC5969
CAS No.:117464-70-7
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- Prionitin
Catalog No.:BCN4855
CAS No.:117469-56-4
The role of endothelin in the control of adrenocortical function: stimulation of endothelin release by ACTH and the effects of endothelin-1 and endothelin-3 on steroidogenesis in rat and human adrenocortical cells.[Pubmed:1848586]
J Endocrinol. 1991 Feb;128(2):275-80.
The rate of blood flow through the intact adrenal gland is closely linked to steroid hormone secretion, and although the mechanism involved is unknown, it is thought to involve secretory products of the vascular endothelium. In dispersed cell preparations, endothelin-1 and -3 both caused a dose-dependent and highly sensitive increase in steroid secretion by zona glomerulosa and zona fasciculata cells of the rat and human adrenal cortex. In addition, when the perfused rat adrenal was stimulated with ACTH, significant increases in steroid secretion and perfusion medium flow rate were accompanied by significantly increased secretion of immunoreactive endothelin into the adrenal vein. It is proposed that endothelin has a role in mediating the adrenocortical response to ACTH stimulation.
Involvement of ET(A) and ET(B) receptors in the activation of phospholipase D by endothelins in cultured rat cortical astrocytes.[Pubmed:9756390]
Br J Pharmacol. 1998 Aug;124(8):1728-34.
This study was performed to characterize the receptor subtypes involved in the endothelin stimulation of phospholipase D (PLD) in rat cortical astrocytes in primary culture. PLD activity was determined by measuring the formation of [32P]phosphatidylbutanol in [32P]orthophosphate prelabelled cells stimulated in the presence of 25 mM butanol. The agonists endothelin-1 (ET-1), endothelin-3 (ET-3), sarafotoxin 6c (S6c) and IRL 1620 elicited PLD activation in a concentration-dependent manner. The potencies of ET-1, ET-3 and S6c were similar. The maximal effects evoked by the ET(B)-preferring agonists, ET-3, S6c and IRL 1620, were significantly lower than the maximal response to the non-selective agonist ET-1. The response to 1 nM ET-1 was inhibited by increasing concentrations of the ET(A) receptor antagonist BQ-123 in a biphasic manner. A high potency component of the inhibition curve (24.2+/-3.5% of the ET-1 response) was defined at low (up to 1 microM) concentrations of BQ-123, yielding an estimated Ki value for BQ-123 of 21.3+/-2.5 nM. In addition, the presence of 1 microM BQ-123 significantly reduced the maximal response to ET-1 but did not change the pD2 value. Increasing concentrations of the ET(B) selective antagonist BQ-788 inhibited the S6c response with a Ki of 17.8+/-0.8 nM. BQ-788 also inhibited the effect of ET-1, although, in this case, two components were defined, accounting for approximately 50% of the response, and showing Ki values of 20.9+/-5.1 nM and 439+/-110 nM, respectively. The ET-1 concentration-response curve was shifted to the right by 1 microM BQ-788, also revealing two components. Only one of them, corresponding to 69.8+/-4.4% of the response, was sensitive to BQ-788 which showed a Ki value of 28.8+/-8.9 nM. Rapid desensitization was achieved by preincubation with ET-1 or S6c. In cells pretreated with S6c neither ET-3 nor S6c activated PLD, but ET-1 still induced approximately 40% of the response shown by non-desensitised cells. This remaining response was insensitive to BQ-788, but fully inhibited by BQ-123. In conclusion, endothelins activate PLD in rat cortical astrocytes acting through both ET(A) and ET(B) receptors, and this response desensitizes rapidly in an apparently homologous fashion. The percentage contribution of ET(A) and ET(B) receptors to the ET-1 response was found to be approximately 20% and 80%, respectively, when ET(B) receptors were not blocked, and 30-50% and 50-70%, respectively, when ET(B) receptors were inhibited or desensitized. These results may be relevant to the study of a possible role of PLD in the proliferative effects shown by endothelins on cultured and reactive astrocytes.
The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes.[Pubmed:2649896]
Proc Natl Acad Sci U S A. 1989 Apr;86(8):2863-7.
Three distinct human endothelin-related genes were cloned by screening a genomic DNA library under a low hybridization stringency with a synthetic oligonucleotide probe encoding a portion of the endothelin sequence. Genomic Southern blot analysis with the same oligonucleotide probe showed three corresponding chromosomal loci not only in the human genome but also in porcine and rat genomes. The nucleotide sequences of the three human genes were highly conserved within the regions encoding the 21-residue (mature) endothelins, in spite of the fact that the immediately upstream exon sequences, which encode a part of the propeptides, retained little similarity. Moreover, each of the human genes predicted a putative 21-residue peptide, similar to but distinct from each other: (i) the "classical" endothelin (ET-1), (ii) [Trp6,Leu7]endothelin (ET-2), and (iii) [Thr2,Phe4,Thr5,Tyr6, Lys7,Tyr14]endothelin (ET-3). Synthetic ET-1, ET-2, and ET-3 were prepared according to the deduced amino acid sequences, and the biological activities were assayed by contraction of isolated porcine coronary artery strips and by intravenous injection to anesthetized rats. All these synthetic peptides produced strong vasoconstrictor and pressor responses. However, the quantitative profiles of the pharmacological activities were considerably different among the three isopeptides, suggesting the possible existence of endothelin receptor subtypes.


